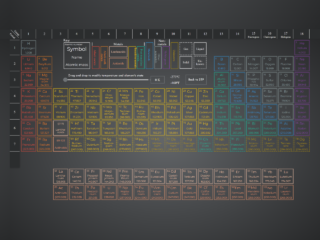
Are you looking to display a clear and interactive periodic table on your website? Creating a responsive and visually appealing Periodic Table of Elements HTML Code can greatly enhance educational content or scientific projects. This tutorial will guide you through building a dynamic periodic table, complete with element details and an interactive temperature slider, using clean HTML, CSS, and JavaScript. It’s perfect for anyone wanting to integrate complex chemical information into a user-friendly web interface.
How to Create a Periodic Table of Elements
Add Header Assets
To begin, include the necessary stylesheet in the `<head>` section of your HTML document. This link provides basic styling and resets for consistent rendering across browsers.
<link rel="stylesheet" href="https://public.codepenassets.com/css/reset-2.0.min.css">
Create the HTML Structure
Next, define the foundational structure of your periodic table. This HTML setup includes the grid for the elements, labels for groups and periods, a legend for classification, a temperature control, and a modal for displaying detailed element information.
<main>
<section class="group-period">
<ul class="group__list">
<li class="group__item flex-row-wrap group-1"><span>1</span></li>
<li class="group__item flex-row-wrap group-2"><span>2</span></li>
<li class="group__item flex-row-wrap group-3"><span>3</span></li>
<li class="group__item flex-row-wrap group-4"><span>4</span></li>
<li class="group__item flex-row-wrap group-5"><span>5</span></li>
<li class="group__item flex-row-wrap group-6"><span>6</span></li>
<li class="group__item flex-row-wrap group-7"><span>7</span></li>
<li class="group__item flex-row-wrap group-8"><span>8</span></li>
<li class="group__item flex-row-wrap group-9"><span>9</span></li>
<li class="group__item flex-row-wrap group-10"><span>10</span></li>
<li class="group__item flex-row-wrap group-11"><span>11</span></li>
<li class="group__item flex-row-wrap group-12"><span>12</span></li>
<li class="group__item flex-row-wrap group-13"><span>13</span></li>
<li class="group__item flex-row-wrap group-14"><span>14</span></li>
<li class="group__item flex-row-wrap group-15"><span>15</span><span>Pnictogens</span></li>
<li class="group__item flex-row-wrap group-16"><span>16</span><span>Chalcogens</span></li>
<li class="group__item flex-row-wrap group-17"><span>17</span><span>Halogens</span></li>
<li class="group__item flex-row-wrap group-18"><span>18</span></li>
</ul>
<ul class="period__list">
<li class="period__item flex-row-wrap period-1"><span>1</span></li>
<li class="period__item flex-row-wrap period-2"><span>2</span></li>
<li class="period__item flex-row-wrap period-3"><span>3</span></li>
<li class="period__item flex-row-wrap period-4"><span>4</span></li>
<li class="period__item flex-row-wrap period-5"><span>5</span></li>
<li class="period__item flex-row-wrap period-6"><span>6</span></li>
<li class="period__item flex-row-wrap period-7"><span>7</span></li>
</ul>
</section>
<section class="dynamic-periodic-table">
<div class="key flex-row-wrap">
<abbr class="key__abbr">Symbol</abbr>
<span class="key__name">Name</span>
<span class="key__atomic-number">Atomic number</span>
<span class="key__atomic-mass">Atomic mass</span>
</div>
<section class="legend flex-row-nowrap">
<article class="legend__element-type flex-row-wrap">
<div class="legend__element-type__metals flex-row-nowrap">
<div class="legend__element-type__metals__alkali-metal legend-box flex-column-wrap" data-element-type="alkali-metal"><span>Alkali metals</span></div>
<div class="legend__element-type__metals__alkali-earth-metal legend-box flex-column-wrap" data-element-type="alkali-earth-metal"><span>Alkali earth metals</span></div>
<div class="legend__element-type__metals__lanthanoid-actinoid flex-row-wrap">
<div class="lanthanoid flex-row-wrap legend-box" data-element-type="Lanthanoid"><span>Lanthanoids</span></div>
<div class="actinoid flex-row-wrap legend-box" data-element-type="Actinoid"><span>Actinoids</span></div>
</div>
<div class="legend__element-type__metals__transition-metal legend-box flex-column-wrap" data-element-type="transition-metal"><span>Transition metals</span></div>
<div class="legend__element-type__metals__post-transition-metal legend-box flex-column-wrap" data-element-type="post-transition-metal"><span>Post-transition metals</span></div>
</div>
<div class="legend__element-type__metalloid legend-box" data-element-type="metalloid"><span>Metalloids</span></div>
<div class="legend__element-type__nonmetals flex-row-wrap">
<div class="legend__element-type__nonmetals__other-nonmetal legend-box" data-element-type="other-nonmetal"><span>Other <br> nonmetals</span></div>
<div class="legend__element-type__nonmetals__noble-gas legend-box" data-element-type="noble-gas"><span>Noble gases</span></div>
</div>
<div class="legend__element-type__unknown legend-box flex-row-wrap" data-element-type="unknown"><span>Unknown</span>
</div>
</article>
<article class="legend__element-state flex-row-wrap">
<div class="legend__element-state__gas legend-box flex-row-wrap" data-element-state="gas"><span>Gas</span></div>
<div class="legend__element-state__liquid legend-box flex-row-wrap" data-element-state="liquid"><span>Liquid</span></div>
<div class="legend__element-state__solid legend-box flex-row-wrap" data-element-state="solid"><span>Solid</span></div>
<div class="legend__element-state__unknown legend-box flex-row-wrap" data-element-state="unknown"><span>Un-known</span></div>
</article>
</section>
<section class="temperature flex-row-nowrap">
<div class="temperature__inputs flex-row-nowrap">
<input class="temperature__inputs__slider" name="temperature__inputs__slider" type="range" min="0" max="7000" value="273">
<p class="temperature__inputs__result flex-row-wrap">273 K</p>
</div>
<div class="temperature__unit-conversion flex-row-wrap">
<p class="celsius">0ºC</p>
<p class="farenheit">31.4ºF</p>
</div>
<button class="reset__temperature flex-row-wrap"><span>Back to STP</span></button>
</section>
<div class="element flex-row-wrap" data-element-type="other-nonmetal" data-element-state="gas" data-element-melting-point="13.99" data-element-boiling-point="20.271" data-element-period="1" data-element-group="1" data-element-name="Hydrogen">
<abbr class="element__abbr">H</abbr>
<span class="element__name">Hydrogen</span>
<span class="element__atomic-number">1</span>
<span class="element__atomic-mass">1.008</span>
<p class="tooltip right-tooltip">Hydrogen</p>
</div>
<div class="element flex-row-wrap" data-element-type="alkali-metal" data-element-state="solid" data-element-melting-point="453.65" data-element-boiling-point="1603" data-element-period="2" data-element-group="1" data-element-name="Lithium">
<abbr class="element__abbr">Li</abbr>
<span class="element__name">Lithium</span>
<span class="element__atomic-number">3</span>
<span class="element__atomic-mass">6.940</span>
<p class="tooltip top-tooltip">Lithium</p>
</div>
<div class="element flex-row-wrap" data-element-type="alkali-metal" data-element-state="solid" data-element-melting-point="370.944" data-element-boiling-point="1156.090" data-element-period="3" data-element-group="1" data-element-name="Sodium">
<abbr class="element__abbr">Na</abbr>
<span class="element__name">Sodium</span>
<span class="element__atomic-number">11</span>
<span class="element__atomic-mass">22.989</span>
<p class="tooltip top-tooltip">Sodium</p>
</div>
<div class="element flex-row-wrap" data-element-type="alkali-metal" data-element-state="solid" data-element-melting-point="336.7" data-element-boiling-point="1032" data-element-period="4" data-element-group="1" data-element-name="Potassium">
<abbr class="element__abbr">K</abbr>
<span class="element__name">Potassium</span>
<span class="element__atomic-number">19</span>
<span class="element__atomic-mass">39.098</span>
<p class="tooltip top-tooltip">Potassium</p>
</div>
<div class="element flex-row-wrap" data-element-type="alkali-metal" data-element-state="solid" data-element-melting-point="312.45" data-element-boiling-point="961" data-element-period="5" data-element-group="1" data-element-name="Rubidium">
<abbr class="element__abbr">Rb</abbr>
<span class="element__name">Rubidium</span>
<span class="element__atomic-number">37</span>
<span class="element__atomic-mass">85.468</span>
<p class="tooltip top-tooltip">Rubidium</p>
</div>
<div class="element flex-row-wrap" data-element-type="alkali-metal" data-element-state="solid" data-element-melting-point="301.7" data-element-boiling-point="944" data-element-period="6" data-element-group="1" data-element-name="Caesium">
<abbr class="element__abbr">Cs</abbr>
<span class="element__name">Caesium</span>
<span class="element__atomic-number">55</span>
<span class="element__atomic-mass">132.905</span>
<p class="tooltip top-tooltip">Caesium</p>
</div>
<div class="element flex-row-wrap" data-element-type="alkali-metal" data-element-state="solid" data-element-melting-point="300" data-element-boiling-point="950" data-element-period="7" data-element-group="1" data-element-name="Francium">
<abbr class="element__abbr">Fr</abbr>
<span class="element__name">Francium</span>
<span class="element__atomic-number">87</span>
<span class="element__atomic-mass">(223.000)</span>
<p class="tooltip bottom-tooltip">Francium</p>
</div>
<div class="element flex-row-wrap" data-element-type="alkali-earth-metal" data-element-state="solid" data-element-melting-point="1560" data-element-boiling-point="2742" data-element-period="2" data-element-group="2" data-element-name="Beryllium">
<abbr class="element__abbr">Be</abbr>
<span class="element__name">Beryllium</span>
<span class="element__atomic-number">4</span>
<span class="element__atomic-mass">9.012</span>
<p class="tooltip right-tooltip">Beryllium</p>
</div>
<div class="element flex-row-wrap" data-element-type="alkali-earth-metal" data-element-state="solid" data-element-melting-point="923" data-element-boiling-point="1363" data-element-period="3" data-element-group="2" data-element-name="Magnesium">
<abbr class="element__abbr">Mg</abbr>
<span class="element__name">Magne-sium</span>
<span class="element__atomic-number">12</span>
<span class="element__atomic-mass">24.305</span>
<p class="tooltip right-tooltip">Magnesium</p>
</div>
<div class="element flex-row-wrap" data-element-type="alkali-earth-metal" data-element-state="solid" data-element-melting-point="1115" data-element-boiling-point="1757" data-element-period="4" data-element-group="2" data-element-name="Calcium">
<abbr class="element__abbr">Ca</abbr>
<span class="element__name">Calcium</span>
<span class="element__atomic-number">20</span>
<span class="element__atomic-mass">40.078</span>
<p class="tooltip top-tooltip">Calcium</p>
</div>
<div class="element flex-row-wrap" data-element-type="alkali-earth-metal" data-element-state="solid" data-element-melting-point="1050" data-element-boiling-point="1650" data-element-period="5" data-element-group="2" data-element-name="Stronium">
<abbr class="element__abbr">Sr</abbr>
<span class="element__name">Stronium</span>
<span class="element__atomic-number">38</span>
<span class="element__atomic-mass">87.620</span>
<p class="tooltip top-tooltip">Stronium</p>
</div>
<div class="element flex-row-wrap" data-element-type="alkali-earth-metal" data-element-state="solid" data-element-melting-point="1000" data-element-boiling-point="2118" data-element-period="6" data-element-group="2" data-element-name="Barium">
<abbr class="element__abbr">Ba</abbr>
<span class="element__name">Barium</span>
<span class="element__atomic-number">56</span>
<span class="element__atomic-mass">137.327</span>
<p class="tooltip top-tooltip">Barium</p>
</div>
<div class="element flex-row-wrap" data-element-type="alkali-earth-metal" data-element-state="solid" data-element-melting-point="973" data-element-boiling-point="2010" data-element-period="7" data-element-group="2" data-element-name="Radium">
<abbr class="element__abbr">Ra</abbr>
<span class="element__name">Radium</span>
<span class="element__atomic-number">88</span>
<span class="element__atomic-mass">(226.000)</span>
<p class="tooltip bottom-tooltip">Radium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="1814" data-element-boiling-point="3109" data-element-period="4" data-element-group="3" data-element-name="Scandium">
<abbr class="element__abbr">Sc</abbr>
<span class="element__name">Scandium</span>
<span class="element__atomic-number">21</span>
<span class="element__atomic-mass">44.956</span>
<p class="tooltip top-tooltip">Scandium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="1799" data-element-boiling-point="3203" data-element-period="5" data-element-group="3" data-element-name="Yttrium">
<abbr class="element__abbr">Y</abbr>
<span class="element__name">Yttrium</span>
<span class="element__atomic-number">39</span>
<span class="element__atomic-mass">88.906</span>
<p class="tooltip top-tooltip">Yttrium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-name="Lanthanoids" data-element-melting-point="0" data-element-boiling-point="7001" data-element-name="Lanthanoids">
<span class="element__name">57-71</span>
<span class="element__name">Lantha-noids</span>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-name="Actinoids" data-element-melting-point="0" data-element-boiling-point="7001">
<span class="element__name">89-103</span>
<span class="element__name">Actinoids</span>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="1941" data-element-boiling-point="3560" data-element-period="4" data-element-group="4" data-element-name="Titanium">
<abbr class="element__abbr">Ti</abbr>
<span class="element__name">Titanium</span>
<span class="element__atomic-number">22</span>
<span class="element__atomic-mass">47.867</span>
<p class="tooltip top-tooltip">Titanium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="2128" data-element-boiling-point="4650" data-element-period="5" data-element-group="4" data-element-name="Zirconium">
<abbr class="element__abbr">Zr</abbr>
<span class="element__name">Zirconium</span>
<span class="element__atomic-number">40</span>
<span class="element__atomic-mass">91.224</span>
<p class="tooltip top-tooltip">Zirconium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="2506" data-element-boiling-point="4876" data-element-period="6" data-element-group="4" data-element-name="Hafnium">
<abbr class="element__abbr">Hf</abbr>
<span class="element__name">Hafnium</span>
<span class="element__atomic-number">72</span>
<span class="element__atomic-mass">178.490</span>
<p class="tooltip top-tooltip">Hafnium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="4" data-element-name="Rutherfordium">
<abbr class="element__abbr">Rf</abbr>
<span class="element__name">Ruther-fordium</span>
<span class="element__atomic-number">104</span>
<span class="element__atomic-mass">(267.000)</span>
<p class="tooltip bottom-tooltip">Rutherfordium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="2183" data-element-boiling-point="3680" data-element-period="4" data-element-group="5" data-element-name="Vanadium">
<abbr class="element__abbr">V</abbr>
<span class="element__name">Vanadium</span>
<span class="element__atomic-number">23</span>
<span class="element__atomic-mass">50.942</span>
<p class="tooltip top-tooltip">Vanadium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="2750" data-element-boiling-point="5017" data-element-period="5" data-element-group="5" data-element-name="Niobium">
<abbr class="element__abbr">Nb</abbr>
<span class="element__name">Niobium</span>
<span class="element__atomic-number">41</span>
<span class="element__atomic-mass">92.906</span>
<p class="tooltip top-tooltip">Niobium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="3290" data-element-boiling-point="5731" data-element-period="6" data-element-group="5" data-element-name="Tantalum">
<abbr class="element__abbr">Ta</abbr>
<span class="element__name">Tantalum</span>
<span class="element__atomic-number">73</span>
<span class="element__atomic-mass">180.947</span>
<p class="tooltip top-tooltip">Tantalum</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="5" data-element-name="Dubnium">
<abbr class="element__abbr">Db</abbr>
<span class="element__name">Dubnium</span>
<span class="element__atomic-number">105</span>
<span class="element__atomic-mass">(268.000)</span>
<p class="tooltip bottom-tooltip">Dubnium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="2180" data-element-boiling-point="2944" data-element-period="4" data-element-group="6" data-element-name="Chromium">
<abbr class="element__abbr">Cr</abbr>
<span class="element__name">Chro-mium</span>
<span class="element__atomic-number">24</span>
<span class="element__atomic-mass">51.996</span>
<p class="tooltip top-tooltip">Chromium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="2896" data-element-boiling-point="4912" data-element-period="5" data-element-group="6" data-element-name="Molybdenum">
<abbr class="element__abbr">Mo</abbr>
<span class="element__name">Molyb-denum</span>
<span class="element__atomic-number">42</span>
<span class="element__atomic-mass">95.950</span>
<p class="tooltip top-tooltip">Molyb-denum</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="3695" data-element-boiling-point="6203" data-element-period="6" data-element-group="6" data-element-name="Tungsten">
<abbr class="element__abbr">W</abbr>
<span class="element__name">Tungsten</span>
<span class="element__atomic-number">74</span>
<span class="element__atomic-mass">183.840</span>
<p class="tooltip top-tooltip">Tungsten</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="6" data-element-name="Seaborgium">
<abbr class="element__abbr">Sg</abbr>
<span class="element__name">Seabor-gium</span>
<span class="element__atomic-number">106</span>
<span class="element__atomic-mass">(268.000)</span>
<p class="tooltip bottom-tooltip">Seaborgium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="1519" data-element-boiling-point="2334" data-element-period="4" data-element-group="7" data-element-name="Manganese">
<abbr class="element__abbr">Mn</abbr>
<span class="element__name">Manga-nese</span>
<span class="element__atomic-number">25</span>
<span class="element__atomic-mass">54.938</span>
<p class="tooltip top-tooltip">Manganese</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="2430" data-element-boiling-point="4538" data-element-period="5" data-element-group="7" data-element-name="Technetium">
<abbr class="element__abbr">Tc</abbr>
<span class="element__name">Techne-tium</span>
<span class="element__atomic-number">43</span>
<span class="element__atomic-mass">(98.000)</span>
<p class="tooltip top-tooltip">Techne-tium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="3459" data-element-boiling-point="5903" data-element-period="6" data-element-group="7" data-element-name="Rhenium">
<abbr class="element__abbr">Re</abbr>
<span class="element__name">Rhenium</span>
<span class="element__atomic-number">75</span>
<span class="element__atomic-mass">186.207</span>
<p class="tooltip top-tooltip">Rhenium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="7" data-element-name="Bohrium">
<abbr class="element__abbr">Bh</abbr>
<span class="element__name">Bohrium</span>
<span class="element__atomic-number">107</span>
<span class="element__atomic-mass">(270.000)</span>
<p class="tooltip bottom-tooltip">Bohrium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="1811" data-element-boiling-point="3134" data-element-period="4" data-element-group="8" data-element-name="Iron">
<abbr class="element__abbr">Fe</abbr>
<span class="element__name">Iron</span>
<span class="element__atomic-number">26</span>
<span class="element__atomic-mass">55.845</span>
<p class="tooltip top-tooltip">Iron</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="2607" data-element-boiling-point="4423" data-element-period="5" data-element-group="8" data-element-name="Ruthenium">
<abbr class="element__abbr">Ru</abbr>
<span class="element__name">Ruthe-nium</span>
<span class="element__atomic-number">44</span>
<span class="element__atomic-mass">101.070</span>
<p class="tooltip top-tooltip">Ruthe-nium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="3306" data-element-boiling-point="5285" data-element-period="6" data-element-group="8" data-element-name="Osmium">
<abbr class="element__abbr">Os</abbr>
<span class="element__name">Osmium</span>
<span class="element__atomic-number">76</span>
<span class="element__atomic-mass">190.230</span>
<p class="tooltip top-tooltip">Osmium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="8" data-element-name="Hassium">
<abbr class="element__abbr">Hs</abbr>
<span class="element__name">Hassium</span>
<span class="element__atomic-number">108</span>
<span class="element__atomic-mass">(277.000)</span>
<p class="tooltip bottom-tooltip">Hassium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="1768" data-element-boiling-point="3200" data-element-period="4" data-element-group="9" data-element-name="Cobalt">
<abbr class="element__abbr">Co</abbr>
<span class="element__name">Cobalt</span>
<span class="element__atomic-number">27</span>
<span class="element__atomic-mass">58.993</span>
<p class="tooltip top-tooltip">Cobalt</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="2237" data-element-boiling-point="3968" data-element-period="5" data-element-group="9" data-element-name="Rhodium">
<abbr class="element__abbr">Rh</abbr>
<span class="element__name">Rhodium</span>
<span class="element__atomic-number">45</span>
<span class="element__atomic-mass">102.905</span>
<p class="tooltip top-tooltip">Rhodium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="2719" data-element-boiling-point="4403" data-element-period="6" data-element-group="9" data-element-name="Iridium">
<abbr class="element__abbr">Ir</abbr>
<span class="element__name">Iridium</span>
<span class="element__atomic-number">77</span>
<span class="element__atomic-mass">192.217</span>
<p class="tooltip top-tooltip">Iridium</p>
</div>
<div class="element flex-row-wrap" data-element-type="unknown" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="9" data-element-name="Meitnerium">
<abbr class="element__abbr">Mt</abbr>
<span class="element__name">Meitne-rium</span>
<span class="element__atomic-number">109</span>
<span class="element__atomic-mass">(278.000)</span>
<p class="tooltip bottom-tooltip">Meitnerium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="1728" data-element-boiling-point="3003" data-element-period="4" data-element-group="10" data-element-name="Nickel">
<abbr class="element__abbr">Ni</abbr>
<span class="element__name">Nickel</span>
<span class="element__atomic-number">28</span>
<span class="element__atomic-mass">58.963</span>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="1828.05" data-element-boiling-point="3236" data-element-period="5" data-element-group="10" data-element-name="Palladium">
<abbr class="element__abbr">Pd</abbr>
<span class="element__name">Palladium</span>
<span class="element__atomic-number">46</span>
<span class="element__atomic-mass">106.420</span>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="2041.4" data-element-boiling-point="4098" data-element-period="6" data-element-group="10" data-element-name="Platinum">
<abbr class="element__abbr">Pt</abbr>
<span class="element__name">Platinum</span>
<span class="element__atomic-number">78</span>
<span class="element__atomic-mass">195.084</span>
</div>
<div class="element flex-row-wrap" data-element-type="unknown" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="10" data-element-name="Darmstadtium">
<abbr class="element__abbr">Ds</abbr>
<span class="element__name">Darmstad-tium</span>
<span class="element__atomic-number">110</span>
<span class="element__atomic-mass">(281.000)</span>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="1357.77" data-element-boiling-point="2835" data-element-period="4" data-element-group="11" data-element-name="Copper">
<abbr class="element__abbr">Cu</abbr>
<span class="element__name">Copper</span>
<span class="element__atomic-number">29</span>
<span class="element__atomic-mass">63.546</span>
<p class="tooltip top-tooltip">Copper</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="1234.93" data-element-boiling-point="2435" data-element-period="5" data-element-group="11" data-element-name="Silver">
<abbr class="element__abbr">Ag</abbr>
<span class="element__name">Silver</span>
<span class="element__atomic-number">47</span>
<span class="element__atomic-mass">107.868</span>
<p class="tooltip top-tooltip">Silver</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="1337.33" data-element-boiling-point="3243" data-element-period="6" data-element-group="11" data-element-name="Gold">
<abbr class="element__abbr">Au</abbr>
<span class="element__name">Gold</span>
<span class="element__atomic-number">79</span>
<span class="element__atomic-mass">107.868</span>
<p class="tooltip top-tooltip">Gold</p>
</div>
<div class="element flex-row-wrap" data-element-type="unknown" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="11" data-element-name="Roentgenium">
<abbr class="element__abbr">Rg</abbr>
<span class="element__name">Roentge-nium</span>
<span class="element__atomic-number">111</span>
<span class="element__atomic-mass">(282.000)</span>
<p class="tooltip bottom-tooltip">Roentge-nium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="692.68" data-element-boiling-point="1180" data-element-period="4" data-element-group="12" data-element-name="Zinc">
<abbr class="element__abbr">Zn</abbr>
<span class="element__name">Zinc</span>
<span class="element__atomic-number">30</span>
<span class="element__atomic-mass">65.380</span>
<p class="tooltip top-tooltip">Zinc</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="solid" data-element-melting-point="594.22" data-element-boiling-point="1040" data-element-period="5" data-element-group="12" data-element-name="Cadmium">
<abbr class="element__abbr">Cd</abbr>
<span class="element__name">Cadmium</span>
<span class="element__atomic-number">48</span>
<span class="element__atomic-mass">112.414</span>
<p class="tooltip top-tooltip">Cadmium</p>
</div>
<div class="element flex-row-wrap" data-element-type="transition-metal" data-element-state="liquid" data-element-melting-point="234.3210" data-element-boiling-point="629.88" data-element-period="6" data-element-group="12" data-element-name="Mercury">
<abbr class="element__abbr">Hg</abbr>
<span class="element__name">Mercury</span>
<span class="element__atomic-number">80</span>
<span class="element__atomic-mass">200.592</span>
<p class="tooltip top-tooltip">Mercury</p>
</div>
<div class="element flex-row-wrap" data-element-type="post-transition-metal" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="12" data-element-name="Copernicium">
<abbr class="element__abbr">Cn</abbr>
<span class="element__name">Coperni-cium</span>
<span class="element__atomic-number">112</span>
<span class="element__atomic-mass">(285.000)</span>
<p class="tooltip bottom-tooltip">Copernicium</p>
</div>
<div class="element flex-row-wrap" data-element-type="metalloid" data-element-state="solid" data-element-melting-point="2349" data-element-boiling-point="4200" data-element-period="2" data-element-group="13" data-element-name="Boron">
<abbr class="element__abbr">B</abbr>
<span class="element__name">Boron</span>
<span class="element__atomic-number">5</span>
<span class="element__atomic-mass">10.810</span>
<p class="tooltip left-tooltip">Boron</p>
</div>
<div class="element flex-row-wrap" data-element-type="post-transition-metal" data-element-state="solid" data-element-melting-point="933.47" data-element-boiling-point="2743" data-element-period="3" data-element-group="13" data-element-name="Aluminium">
<abbr class="element__abbr">Al</abbr>
<span class="element__name">Alumi-nium</span>
<span class="element__atomic-number">13</span>
<span class="element__atomic-mass">26.982</span>
<p class="tooltip left-tooltip">Aluminium</p>
</div>
<div class="element flex-row-wrap" data-element-type="post-transition-metal" data-element-state="solid" data-element-melting-point="302.9146" data-element-boiling-point="2673" data-element-period="4" data-element-group="13" data-element-name="Galium">
<abbr class="element__abbr">Ga</abbr>
<span class="element__name">Galium</span>
<span class="element__atomic-number">31</span>
<span class="element__atomic-mass">69.723</span>
<p class="tooltip top-tooltip">Galium</p>
</div>
<div class="element flex-row-wrap" data-element-type="post-transition-metal" data-element-state="solid" data-element-melting-point="429.7485" data-element-boiling-point="2345" data-element-period="5" data-element-group="13" data-element-name="Indium">
<abbr class="element__abbr">In</abbr>
<span class="element__name">Indium</span>
<span class="element__atomic-number">49</span>
<span class="element__atomic-mass">114.818</span>
<p class="tooltip top-tooltip">Indium</p>
</div>
<div class="element flex-row-wrap" data-element-type="post-transition-metal" data-element-state="solid" data-element-melting-point="577" data-element-boiling-point="1746" data-element-period="6" data-element-group="13" data-element-name="Thalium">
<abbr class="element__abbr">Th</abbr>
<span class="element__name">Thalium</span>
<span class="element__atomic-number">81</span>
<span class="element__atomic-mass">204.380</span>
<p class="tooltip top-tooltip">Thalium</p>
</div>
<div class="element flex-row-wrap" data-element-type="unknown" data-element-state="unknown" data-element-period="7" data-element-group="13" data-element-name="Nihonium" data-element-melting-point="unknown" data-element-boiling-point="unknown">
<abbr class="element__abbr">Nh</abbr>
<span class="element__name">Nihonium</span>
<span class="element__atomic-number">113</span>
<span class="element__atomic-mass">(286.000)</span>
<p class="tooltip bottom-tooltip">Nihonium</p>
</div>
<div class="element flex-row-wrap" data-element-type="other-nonmetal" data-element-state="solid" data-element-melting-point="3915" data-element-boiling-point="4600" data-element-period="2" data-element-group="14" data-element-name="Carbon">
<abbr class="element__abbr">C</abbr>
<span class="element__name">Carbon</span>
<span class="element__atomic-number">6</span>
<span class="element__atomic-mass">12.001</span>
<p class="tooltip top-tooltip">Carbon</p>
</div>
<div class="element flex-row-wrap" data-element-type="metalloid" data-element-state="solid" data-element-melting-point="1687" data-element-boiling-point="3538" data-element-period="3" data-element-group="14" data-element-name="Silicon">
<abbr class="element__abbr">Si</abbr>
<span class="element__name">Silicon</span>
<span class="element__atomic-number">14</span>
<span class="element__atomic-mass">28.085</span>
<p class="tooltip top-tooltip">Silicon</p>
</div>
<div class="element flex-row-wrap" data-element-type="metalloid" data-element-state="solid" data-element-melting-point="1211.40" data-element-boiling-point="3106" data-element-period="4" data-element-group="14" data-element-name="Germanium">
<abbr class="element__abbr">Ge</abbr>
<span class="element__name">Germa-nium</span>
<span class="element__atomic-number">32</span>
<span class="element__atomic-mass">72.630</span>
<p class="tooltip top-tooltip">Germa-nium</p>
</div>
<div class="element flex-row-wrap" data-element-type="post-transition-metal" data-element-state="solid" data-element-melting-point="505.08" data-element-boiling-point="2875" data-element-period="5" data-element-group="14" data-element-name="Tin">
<abbr class="element__abbr">Sn</abbr>
<span class="element__name">Tin</span>
<span class="element__atomic-number">50</span>
<span class="element__atomic-mass">118.710</span>
<p class="tooltip top-tooltip">Tin</p>
</div>
<div class="element flex-row-wrap" data-element-type="post-transition-metal" data-element-state="solid" data-element-melting-point="600.61" data-element-boiling-point="2022" data-element-period="6" data-element-group="14" data-element-name="Lead">
<abbr class="element__abbr">Pb</abbr>
<span class="element__name">Lead</span>
<span class="element__atomic-number">82</span>
<span class="element__atomic-mass">207.200</span>
<p class="tooltip top-tooltip">Lead</p>
</div>
<div class="element flex-row-wrap" data-element-type="post-transition-metal" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="14" data-element-name="Flerovium">
<abbr class="element__abbr">Fl</abbr>
<span class="element__name">Flerovium</span>
<span class="element__atomic-number">114</span>
<span class="element__atomic-mass">(289.000)</span>
<p class="tooltip bottom-tooltip">Flevorium</p>
</div>
<div class="element flex-row-wrap" data-element-type="other-nonmetal" data-element-state="gas" data-element-melting-point="63.15" data-element-boiling-point="77.355" data-element-period="2" data-element-group="15" data-element-name="Nitrogen">
<abbr class="element__abbr">N</abbr>
<span class="element__name">Nitrogen</span>
<span class="element__atomic-number">7</span>
<span class="element__atomic-mass">14.007</span>
<p class="tooltip top-tooltip">Nitrogen</p>
</div>
<div class="element flex-row-wrap" data-element-type="other-nonmetal" data-element-state="solid" data-element-melting-point="317.2" data-element-boiling-point="553" data-element-period="3" data-element-group="15" data-element-name="Phosphorus">
<abbr class="element__abbr">P</abbr>
<span class="element__name">Phospho-rus</span>
<span class="element__atomic-number">15</span>
<span class="element__atomic-mass">30.974</span>
<p class="tooltip top-tooltip">Phospho-rus</p>
</div>
<div class="element flex-row-wrap" data-element-type="metalloid" data-element-state="solid" data-element-melting-point="887" data-element-boiling-point="887" data-element-period="4" data-element-group="15" data-element-name="Arsenic">
<abbr class="element__abbr">As</abbr>
<span class="element__name">Arsenic</span>
<span class="element__atomic-number">33</span>
<span class="element__atomic-mass">74.922</span>
<p class="tooltip top-tooltip">Arsenic</p>
</div>
<div class="element flex-row-wrap" data-element-type="metalloid" data-element-state="solid" data-element-melting-point="903.78" data-element-boiling-point="1908" data-element-period="5" data-element-group="15" data-element-name="Antimony">
<abbr class="element__abbr">Sb</abbr>
<span class="element__name">Antimony</span>
<span class="element__atomic-number">51</span>
<span class="element__atomic-mass">121.760</span>
<p class="tooltip top-tooltip">Antimony</p>
</div>
<div class="element flex-row-wrap" data-element-type="post-transition-metal" data-element-state="solid" data-element-melting-point="544.7" data-element-boiling-point="1837" data-element-period="6" data-element-group="15" data-element-name="Bismuth">
<abbr class="element__abbr">Bi</abbr>
<span class="element__name">Bismuth</span>
<span class="element__atomic-number">83</span>
<span class="element__atomic-mass">208.980</span>
<p class="tooltip top-tooltip">Bismuth</p>
</div>
<div class="element flex-row-wrap" data-element-type="unknown" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="15" data-element-name="Moscovium">
<abbr class="element__abbr">Mc</abbr>
<span class="element__name">Mosco-vium</span>
<span class="element__atomic-number">115</span>
<span class="element__atomic-mass">(290.000)</span>
<p class="tooltip bottom-tooltip">Moscovium</p>
</div>
<div class="element flex-row-wrap" data-element-type="other-nonmetal" data-element-state="gas" data-element-melting-point="54.36" data-element-boiling-point="90.188" data-element-period="2" data-element-group="16" data-element-name="Oxygen">
<abbr class="element__abbr">O</abbr>
<span class="element__name">Oxygen</span>
<span class="element__atomic-number">8</span>
<span class="element__atomic-mass">15.999</span>
<p class="tooltip top-tooltip">Oxygen</p>
</div>
<div class="element flex-row-wrap" data-element-type="other-nonmetal" data-element-state="solid" data-element-melting-point="54.36" data-element-boiling-point="717.8" data-element-period="3" data-element-group="16" data-element-name="Sulfur">
<abbr class="element__abbr">S</abbr>
<span class="element__name">Sulfur</span>
<span class="element__atomic-number">16</span>
<span class="element__atomic-mass">32.060</span>
<p class="tooltip top-tooltip">Sulfur</p>
</div>
<div class="element flex-row-wrap" data-element-type="other-nonmetal" data-element-state="solid" data-element-melting-point="494" data-element-boiling-point="958" data-element-period="4" data-element-group="16" data-element-name="Selenium">
<abbr class="element__abbr">Se</abbr>
<span class="element__name">Selenium</span>
<span class="element__atomic-number">34</span>
<span class="element__atomic-mass">78.971</span>
<p class="tooltip top-tooltip">Selenium</p>
</div>
<div class="element flex-row-wrap" data-element-type="metalloid" data-element-state="solid" data-element-melting-point="722.66" data-element-boiling-point="1261" data-element-period="5" data-element-group="16" data-element-name="Tellurium">
<abbr class="element__abbr">Te</abbr>
<span class="element__name">Tellurium</span>
<span class="element__atomic-number">52</span>
<span class="element__atomic-mass">127.600</span>
<p class="tooltip top-tooltip">Tellurium</p>
</div>
<div class="element flex-row-wrap" data-element-type="post-transition-metal" data-element-state="solid" data-element-melting-point="527" data-element-boiling-point="1235" data-element-period="6" data-element-group="16" data-element-name="Polonium">
<abbr class="element__abbr">Po</abbr>
<span class="element__name">Polonium</span>
<span class="element__atomic-number">84</span>
<span class="element__atomic-mass">(209.000)</span>
<p class="tooltip top-tooltip">Polonium</p>
</div>
<div class="element flex-row-wrap" data-element-type="unknown" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="16" data-element-name="Livemorium">
<abbr class="element__abbr">Lv</abbr>
<span class="element__name">Livemo-rium</span>
<span class="element__atomic-number">116</span>
<span class="element__atomic-mass">(293.000)</span>
<p class="tooltip bottom-tooltip">Livemorium</p>
</div>
<div class="element flex-row-wrap" data-element-type="other-nonmetal" data-element-state="gas" data-element-melting-point="53.48" data-element-boiling-point="85.03" data-element-period="2" data-element-group="17" data-element-name="Fluorine">
<abbr class="element__abbr">F</abbr>
<span class="element__name">Fluorine</span>
<span class="element__atomic-number">9</span>
<span class="element__atomic-mass">18.998</span>
<p class="tooltip top-tooltip">Fluorine</p>
</div>
<div class="element flex-row-wrap" data-element-type="other-nonmetal" data-element-state="gas" data-element-melting-point="171.6" data-element-boiling-point="239.11" data-element-period="3" data-element-group="17" data-element-name="Chlorine">
<abbr class="element__abbr">Cl</abbr>
<span class="element__name">Chlorine</span>
<span class="element__atomic-number">17</span>
<span class="element__atomic-mass">35.450</span>
<p class="tooltip top-tooltip">Chlorine</p>
</div>
<div class="element flex-row-wrap" data-element-type="other-nonmetal" data-element-state="liquid" data-element-melting-point="265.8" data-element-boiling-point="332" data-element-period="4" data-element-group="17" data-element-name="Bromine">
<abbr class="element__abbr">Br</abbr>
<span class="element__name">Bromine</span>
<span class="element__atomic-number">35</span>
<span class="element__atomic-mass">79.904</span>
<p class="tooltip top-tooltip">Bromine</p>
</div>
<div class="element flex-row-wrap" data-element-type="other-nonmetal" data-element-state="solid" data-element-melting-point="386.85" data-element-boiling-point="457.4" data-element-period="5" data-element-group="17" data-element-name="Iodine">
<abbr class="element__abbr">I</abbr>
<span class="element__name">Iodine</span>
<span class="element__atomic-number">53</span>
<span class="element__atomic-mass">126.904</span>
<p class="tooltip top-tooltip">Iodine</p>
</div>
<div class="element flex-row-wrap" data-element-type="metalloid" data-element-state="solid" data-element-melting-point="575" data-element-boiling-point="610" data-element-period="7" data-element-group="17" data-element-name="Astatine">
<abbr class="element__abbr">At</abbr>
<span class="element__name">Astatine</span>
<span class="element__atomic-number">85</span>
<span class="element__atomic-mass">(210.000)</span>
<p class="tooltip top-tooltip">Astatine</p>
</div>
<div class="element flex-row-wrap" data-element-type="unknown" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="17" data-element-name="Tennessine">
<abbr class="element__abbr">Ts</abbr>
<span class="element__name">Tennes-sine</span>
<span class="element__atomic-number">117</span>
<span class="element__atomic-mass">(294.000)</span>
<p class="tooltip bottom-tooltip">Tennessine</p>
</div>
<div class="element flex-row-wrap" data-element-type="noble-gas" data-element-state="gas" data-element-melting-point="0.95" data-element-boiling-point="4.222" data-element-period="1" data-element-group="18" data-element-name="Helium">
<abbr class="element__abbr">He</abbr>
<span class="element__name">Helium</span>
<span class="element__atomic-number">2</span>
<span class="element__atomic-mass">4.002</span>
<p class="tooltip left-tooltip">Helium</p>
</div>
<div class="element flex-row-wrap" data-element-type="noble-gas" data-element-state="gas" data-element-melting-point="24.56" data-element-boiling-point="27.104" data-element-period="2" data-element-group="18" data-element-name="Neon">
<abbr class="element__abbr">Ne</abbr>
<span class="element__name">Neon</span>
<span class="element__atomic-number">10</span>
<span class="element__atomic-mass">20.180</span>
<p class="tooltip top-tooltip">Neon</p>
</div>
<div class="element flex-row-wrap" data-element-type="noble-gas" data-element-state="gas" data-element-melting-point="83.81" data-element-boiling-point="87.302" data-element-period="3" data-element-group="18" data-element-name="Argon">
<abbr class="element__abbr">Ar</abbr>
<span class="element__name">Argon</span>
<span class="element__atomic-number">18</span>
<span class="element__atomic-mass">39.948</span>
<p class="tooltip top-tooltip">Argon</p>
</div>
<div class="element flex-row-wrap" data-element-type="noble-gas" data-element-state="gas" data-element-melting-point="115.78" data-element-boiling-point="119.93" data-element-period="4" data-element-group="18" data-element-name="Krypton">
<abbr class="element__abbr">Kr</abbr>
<span class="element__name">Krypton</span>
<span class="element__atomic-number">36</span>
<span class="element__atomic-mass">83.798</span>
<p class="tooltip top-tooltip">Krypton</p>
</div>
<div class="element flex-row-wrap" data-element-type="noble-gas" data-element-state="gas" data-element-melting-point="161.40" data-element-boiling-point="165.051" data-element-period="5" data-element-group="18" data-element-name="Xenon">
<abbr class="element__abbr">Xe</abbr>
<span class="element__name">Xenon</span>
<span class="element__atomic-number">54</span>
<span class="element__atomic-mass">131.293</span>
<p class="tooltip top-tooltip">Xenon</p>
</div>
<div class="element flex-row-wrap" data-element-type="noble-gas" data-element-state="gas" data-element-melting-point="202" data-element-boiling-point="211.5" data-element-period="6" data-element-group="18" data-element-name="Radon">
<abbr class="element__abbr">Rn</abbr>
<span class="element__name">Radon</span>
<span class="element__atomic-number">86</span>
<span class="element__atomic-mass">(222.000)</span>
<p class="tooltip top-tooltip">Radon</p>
</div>
<div class="element flex-row-wrap" data-element-type="unknown" data-element-state="unknown" data-element-melting-point="unknown" data-element-boiling-point="unknown" data-element-period="7" data-element-group="18" data-element-symbol="Og" data-element-name="Oganesson">
<abbr class="element__abbr">Og</abbr>
<span class="element__name">Oganes-son</span>
<span class="element__atomic-number">118</span>
<span class="element__atomic-mass">(294.000)</span>
<p class="tooltip bottom-tooltip">Oganesson</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1193" data-element-boiling-point="3737" data-element-period="6" data-element-name="Lanthanum">
<abbr class="element__abbr">La</abbr>
<span class="element__name">Lantha-num</span>
<span class="element__atomic-number">57</span>
<span class="element__atomic-mass">138.905</span>
<p class="tooltip top-tooltip">Lanthanum</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1068" data-element-boiling-point="3716" data-element-period="6" data-element-name="Cerium">
<abbr class="element__abbr">Ce</abbr>
<span class="element__name">Cerium</span>
<span class="element__atomic-number">58</span>
<span class="element__atomic-mass">140.116</span>
<p class="tooltip top-tooltip">Cerium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1208" data-element-boiling-point="3403" data-element-period="6" data-element-name="Praseodymium">
<abbr class="element__abbr">Pr</abbr>
<span class="element__name">Praseod-ymium</span>
<span class="element__atomic-number">59</span>
<span class="element__atomic-mass">140.907</span>
<p class="tooltip top-tooltip">Praseodymium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1297" data-element-boiling-point="3347" data-element-period="6" data-element-name="Neodymium">
<abbr class="element__abbr">Nd</abbr>
<span class="element__name">Neod-ymium</span>
<span class="element__atomic-number">60</span>
<span class="element__atomic-mass">144.242</span>
<p class="tooltip top-tooltip">Neodymium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1315" data-element-boiling-point="3273" data-element-period="6" data-element-name="Promethium">
<abbr class="element__abbr">Pm</abbr>
<span class="element__name">Prome-thium</span>
<span class="element__atomic-number">61</span>
<span class="element__atomic-mass">(145,000)</span>
<p class="tooltip top-tooltip">Promethium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1345" data-element-boiling-point="2173" data-element-period="6" data-element-name="Samarium">
<abbr class="element__abbr">Sm</abbr>
<span class="element__name">Samarium</span>
<span class="element__atomic-number">62</span>
<span class="element__atomic-mass">150.360</span>
<p class="tooltip top-tooltip">Samarium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1099" data-element-boiling-point="1802" data-element-period="6" data-element-name="Europium">
<abbr class="element__abbr">Eu</abbr>
<span class="element__name">Europium</span>
<span class="element__atomic-number">63</span>
<span class="element__atomic-mass">151.964</span>
<p class="tooltip top-tooltip">Europium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1585" data-element-boiling-point="3273" data-element-period="6" data-element-name="Gadolinium">
<abbr class="element__abbr">Gd</abbr>
<span class="element__name">Gadoli-nium</span>
<span class="element__atomic-number">64</span>
<span class="element__atomic-mass">157.250</span>
<p class="tooltip top-tooltip">Gadolinium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1629" data-element-boiling-point="3396" data-element-period="6" data-element-name="Terbium">
<abbr class="element__abbr">Tb</abbr>
<span class="element__name">Terbium</span>
<span class="element__atomic-number">65</span>
<span class="element__atomic-mass">157.250</span>
<p class="tooltip top-tooltip">Terbium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1680" data-element-boiling-point="2840" data-element-period="6" data-element-name="Dysprosium">
<abbr class="element__abbr">Dy</abbr>
<span class="element__name">Dyspro-sium</span>
<span class="element__atomic-number">66</span>
<span class="element__atomic-mass">162.500</span>
<p class="tooltip top-tooltip">Dysprosium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1734" data-element-boiling-point="2873" data-element-period="6" data-element-name="Holmium">
<abbr class="element__abbr">Ho</abbr>
<span class="element__name">Holmium</span>
<span class="element__atomic-number">67</span>
<span class="element__atomic-mass">164.930</span>
<p class="tooltip top-tooltip">Holmium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1802" data-element-boiling-point="3141" data-element-period="6" data-element-name="Erbium">
<abbr class="element__abbr">Er</abbr>
<span class="element__name">Erbium</span>
<span class="element__atomic-number">68</span>
<span class="element__atomic-mass">167.259</span>
<p class="tooltip top-tooltip">Erbium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1818" data-element-boiling-point="2223" data-element-period="6" data-element-name="Thulium">
<abbr class="element__abbr">Tm</abbr>
<span class="element__name">Thulium</span>
<span class="element__atomic-number">69</span>
<span class="element__atomic-mass">168.934</span>
<p class="tooltip top-tooltip">Thulium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1097" data-element-boiling-point="1469" data-element-period="6" data-element-name="Ytterbium">
<abbr class="element__abbr">Yb</abbr>
<span class="element__name">Ytterbium</span>
<span class="element__atomic-number">70</span>
<span class="element__atomic-mass">173.045</span>
<p class="tooltip top-tooltip">Ytterbium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Lanthanoid" data-element-state="solid" data-element-melting-point="1925" data-element-boiling-point="3675" data-element-period="6" data-element-name="Lutetium">
<abbr class="element__abbr">Lu</abbr>
<span class="element__name">Lutetium</span>
<span class="element__atomic-number">71</span>
<span class="element__atomic-mass">174.967</span>
<p class="tooltip top-tooltip">Lutetium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="1500" data-element-boiling-point="3500" data-element-period="7" data-element-name="Actinium">
<abbr class="element__abbr">Ac</abbr>
<span class="element__name">Actinium</span>
<span class="element__atomic-number">89</span>
<span class="element__atomic-mass">(227.000)</span>
<p class="tooltip bottom-tooltip">Actinium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="2023" data-element-boiling-point="5061" data-element-period="7" data-element-name="Thorium">
<abbr class="element__abbr">Th</abbr>
<span class="element__name">Thorium</span>
<span class="element__atomic-number">90</span>
<span class="element__atomic-mass">232.038</span>
<p class="tooltip bottom-tooltip">Thorium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="1841" data-element-boiling-point="4300" data-element-period="7" data-element-name="Protactinium">
<abbr class="element__abbr">Pa</abbr>
<span class="element__name">Protacti-nium</span>
<span class="element__atomic-number">91</span>
<span class="element__atomic-mass">231.035</span>
<p class="tooltip bottom-tooltip">Protactinium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="1405.3" data-element-boiling-point="4404" data-element-period="7" data-element-name="Uranium">
<abbr class="element__abbr">U</abbr>
<span class="element__name">Uranium</span>
<span class="element__atomic-number">92</span>
<span class="element__atomic-mass">238.028</span>
<p class="tooltip bottom-tooltip">Uranium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="912" data-element-boiling-point="4447" data-element-period="7" data-element-name="Neptunium">
<abbr class="element__abbr">Np</abbr>
<span class="element__name">Neptu-nium</span>
<span class="element__atomic-number">93</span>
<span class="element__atomic-mass">(237.000)</span>
<p class="tooltip bottom-tooltip">Neptunium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="912.5" data-element-boiling-point="3505" data-element-period="7" data-element-name="Plutonium">
<abbr class="element__abbr">Pu</abbr>
<span class="element__name">Pluto-nium</span>
<span class="element__atomic-number">94</span>
<span class="element__atomic-mass">(244.000)</span>
<p class="tooltip bottom-tooltip">Plutonium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="1449" data-element-boiling-point="2880" data-element-period="7" data-element-name="Americium">
<abbr class="element__abbr">Am</abbr>
<span class="element__name">Ameri-cium</span>
<span class="element__atomic-number">95</span>
<span class="element__atomic-mass">(243.000)</span>
<p class="tooltip bottom-tooltip">Americium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="1613" data-element-boiling-point="3383" data-element-period="7" data-element-name="Curium">
<abbr class="element__abbr">Cm</abbr>
<span class="element__name">Curium</span>
<span class="element__atomic-number">96</span>
<span class="element__atomic-mass">(247.000)</span>
<p class="tooltip bottom-tooltip">Curium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="1259" data-element-boiling-point="2900" data-element-period="7" data-element-name="Berkelium">
<abbr class="element__abbr">Bk</abbr>
<span class="element__name">Berkelium</span>
<span class="element__atomic-number">97</span>
<span class="element__atomic-mass">(247.000)</span>
<p class="tooltip bottom-tooltip">Berkelium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="1173" data-element-boiling-point="1743" data-element-period="7" data-element-name="Californium">
<abbr class="element__abbr">Cf</abbr>
<span class="element__name">Califor-nium</span>
<span class="element__atomic-number">98</span>
<span class="element__atomic-mass">(251.000)</span>
<p class="tooltip bottom-tooltip">Californium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="1133" data-element-boiling-point="1269" data-element-period="7" data-element-name="Einsteinium">
<abbr class="element__abbr">Es</abbr>
<span class="element__name">Einstei-nium</span>
<span class="element__atomic-number">99</span>
<span class="element__atomic-mass">(252.000)</span>
<p class="tooltip bottom-tooltip">Einsteinium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="1133" data-element-boiling-point="1269" data-element-period="7" data-element-name="Fermium">
<abbr class="element__abbr">Fm</abbr>
<span class="element__name">Fermium</span>
<span class="element__atomic-number">100</span>
<span class="element__atomic-mass">(257.000)</span>
<p class="tooltip bottom-tooltip">Fermium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="1133" data-element-boiling-point="unknown" data-element-period="7" data-element-name="Mendelevium">
<abbr class="element__abbr">Md</abbr>
<span class="element__name">Mendele-vium</span>
<span class="element__atomic-number">101</span>
<span class="element__atomic-mass">(258.000)</span>
<p class="tooltip bottom-tooltip">Mendelevium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="1100" data-element-boiling-point="unknown" data-element-period="7" data-element-name="Nobelium">
<abbr class="element__abbr">No</abbr>
<span class="element__name">Nobelium</span>
<span class="element__atomic-number">102</span>
<span class="element__atomic-mass">(259.000)</span>
<p class="tooltip bottom-tooltip">Nobelium</p>
</div>
<div class="element flex-row-wrap" data-element-type="Actinoid" data-element-state="solid" data-element-melting-point="1900" data-element-boiling-point="unknown" data-element-period="7" data-element-name="Lawrencium">
<abbr class="element__abbr">Lr</abbr>
<span class="element__name">Lawren-cium</span>
<span class="element__atomic-number">103</span>
<span class="element__atomic-mass">(266.000)</span>
<p class="tooltip bottom-tooltip">Lawrencium</p>
</div>
</section>
<section class="modal flex-row-wrap">
<dialog class="modal__dialog" open>
<article class="modal__content">
<div class="modal__content__properties"></div>
<div class="atom">
<div class="atom__core"></div>
</div>
</article>
<a class="modal__close">
<svg viewBox="0 0 24 24">
<path d="M19 6.41l-1.41-1.41-5.59 5.59-5.59-5.59-1.41 1.41 5.59 5.59-5.59 5.59 1.41 1.41 5.59-5.59 5.59 5.59 1.41-1.41-5.59-5.59z" />
<path d="M0 0h24v24h-24z" fill="none" />
</svg>
</a>
</dialog>
</section>
</main>
<script src="./script.js"></script>
Style with CSS
Now, apply these CSS rules to visually enhance your periodic table. These styles ensure a modern layout, adapt to different screen sizes, and give unique appearances to various element types and states.
@import url("https://fonts.googleapis.com/css?family=Josefin+Sans|Montserrat");
* {
box-sizing: border-box;
}
body {
display: flex;
justify-content: center;
height: 100%;
font-size: 16px;
color: white;
font-family: "Josefin Sans", "Roboto", sans-serif;
overflow: auto;
}
@media screen and (max-width: 1450px) {
body {
justify-content: normal;
}
}
main {
display: flex;
flex-flow: row wrap;
align-content: center;
justify-content: center;
width: 100%;
height: 100dvh;
}
@media screen and (max-width: 1450px) {
main {
justify-content: normal;
}
}
@media screen and (min-aspect-ratio: 1/1) {
main {
height: 150vh;
}
}
@media screen and (min-aspect-ratio: 1/4) {
main {
height: 155vh;
}
}
@media screen and (max-aspect-ratio: 1/5) {
main {
height: 50vw;
}
}
a {
color: inherit;
text-decoration: none;
outline: none;
}
li {
position: relative;
cursor: pointer;
z-index: 10;
}
sup {
vertical-align: super;
font-size: smaller;
}
.flex-row-wrap {
display: flex;
flex-flow: row wrap;
}
.flex-row-nowrap {
display: flex;
flex-flow: row nowrap;
}
.flex-column-nowrap {
display: flex;
flex-flow: column nowrap;
}
.flex-column-wrap {
display: flex;
flex-flow: column wrap;
}
.group-period {
height: 100%;
width: 100%;
position: relative;
min-width: 1450px;
background: radial-gradient(#636669, #252627);
}
.group__list,
.period__list {
background: #2b2d2e;
box-shadow: 0 0 0.5rem #252627;
}
.group__item,
.period__item {
cursor: default;
}
.group__item span,
.period__item span {
transition: all 0.2s ease;
}
.group__item:hover span,
.period__item:hover span {
box-shadow: 0 0 1rem #3b3a3a;
font-size: 1.5rem;
}
.group__list {
position: sticky;
top: 0;
display: grid;
grid-template: repeat(1, 46.6666666667px)/repeat(18, 70px);
grid-gap: 5px;
justify-content: center;
width: 100%;
min-width: 1300px;
height: 46.6666666667px;
margin: 0 auto;
font-size: 1rem;
z-index: 10;
}
.group__list.--is-fixed {
z-index: 20;
}
.group__list.--is-fixed .group-1::before {
display: none;
}
@media screen and (max-width: 1450px) {
.group__list.--is-fixed .group-1::after {
transform: translateX(-25%) rotate(0);
}
}
.group__item {
width: 70px;
justify-content: center;
align-items: center;
border-right: 1px solid white;
}
.group__item span:nth-of-type(2) {
flex-basis: 100%;
text-align: center;
font-size: 0.75rem;
}
.group__item.group-1 {
border-left: 1px solid white;
transition: all 0.2s ease;
}
.group__item.group-1::after {
content: "Group";
position: absolute;
top: 50%;
font-size: 0.75rem;
left: -40px;
transform: translateY(-50%);
cursor: auto;
transition: all 0.2s ease;
}
@media screen and (max-width: 1450px) {
.group__item.group-1::after {
top: 1.25rem;
left: -35px;
transform: translateY(-50%) rotate(45deg);
}
}
.group__item.group-1::before {
transition: all 0.2s ease;
}
@media screen and (max-width: 1450px) {
.group__item.group-1::before {
content: "";
position: absolute;
width: 50px;
height: 1px;
background-color: white;
transform: rotate(45deg);
left: -55px;
}
}
.group__item.group-15 span:nth-of-type(1), .group__item.group-16 span:nth-of-type(1), .group__item.group-17 span:nth-of-type(1) {
margin-top: 0.75rem;
}
.period__list {
position: absolute;
display: grid;
grid-template: repeat(8, 70px)/repeat(1, 46.6666666667px);
grid-gap: 5px;
width: 100%;
min-width: 46.6666666667px;
max-width: 46.6666666667px;
padding-top: 0.4rem;
font-size: 1rem;
}
.period__list.--is-fixed {
z-index: 10;
}
.period__list.--is-fixed .period-1::before {
content: "";
position: absolute;
background: #2b2d2e;
width: 100%;
height: 75%;
box-shadow: 0 0 0.5rem #252627;
transform: translate(0, -125%);
}
.period__list.--is-fixed .period-1::after {
transform: translateX(25%) rotate(0);
}
.period__item {
width: 46.6666666667px;
justify-content: center;
align-items: center;
border-bottom: 1px solid white;
}
.period__item.period-1 {
border-top: 1px solid white;
}
@media screen and (min-width: 1590px) {
.period__item.period-1::before {
content: "";
position: absolute;
background: #2b2d2e;
width: 100%;
height: 75%;
box-shadow: 0 0 0.5rem #252627;
transform: translate(0, -125%);
}
}
.period__item.period-1::after {
content: "Period";
position: absolute;
font-size: 0.75rem;
top: -0.5rem;
transform: translateY(-50%);
cursor: auto;
transition: all 0.2s ease;
}
@media screen and (max-width: 1450px) {
.period__item.period-1::after {
top: -1.25rem;
left: -3px;
transform: translateY(-50%) rotate(45deg);
}
}
.dynamic-periodic-table {
display: grid;
position: absolute;
top: 46.6666666667px;
grid-gap: 5px;
grid-template: repeat(10, 70px)/repeat(18, 70px);
align-self: center;
justify-self: center;
justify-content: center;
width: 100%;
min-width: 1300px;
margin-top: 0.4rem;
}
@media screen and (max-width: 1447.5px) {
.dynamic-periodic-table {
justify-content: normal;
margin-left: 53px;
}
}
.key,
.element {
border: 2px solid;
font-family: "Montserrat", sans-serif;
}
.key__abbr, .key__name,
.element__abbr,
.element__name {
flex-basis: 100%;
text-align: center;
}
.key__name,
.element__name {
text-align: center;
word-break: break-word;
}
.key__abbr,
.element__abbr {
font-size: 1rem;
}
.key__atomic-number,
.element__atomic-number {
position: absolute;
top: 0.2rem;
left: 0.2rem;
font-size: 0.65rem;
}
.key {
position: relative;
justify-content: center;
align-items: center;
grid-area: 1/4/3/5;
align-self: center;
justify-self: center;
width: 122.5px;
height: 122.5px;
border-color: white;
font-size: 1rem;
}
.key__abbr {
font-size: 1.5rem;
margin-top: 0.5rem;
}
.key::after {
content: "Key:";
position: absolute;
top: -1.15rem;
left: -0.2rem;
font-family: "Josefin Sans", "Roboto", sans-serif;
}
.legend {
position: relative;
align-self: center;
justify-self: center;
justify-content: space-between;
align-items: center;
grid-area: 1/7/3/11;
width: 533.75px;
height: 122.5px;
}
.legend__element-type {
width: 402.5px;
justify-content: space-between;
height: 100%;
}
.legend__element-type-box {
cursor: default;
}
.legend__element-type__metals, .legend__element-type__nonmetals {
position: relative;
align-self: flex-end;
justify-content: space-between;
height: 85%;
}
.legend__element-type__metals__alkali-metal, .legend__element-type__metals__alkali-earth-metal, .legend__element-type__metals__transition-metal, .legend__element-type__metals__post-transition-metal, .legend__element-type__nonmetals__alkali-metal, .legend__element-type__nonmetals__alkali-earth-metal, .legend__element-type__nonmetals__transition-metal, .legend__element-type__nonmetals__post-transition-metal {
justify-content: center;
width: 35px;
height: 100%;
text-align: center;
margin-right: 5px;
border: 2px solid;
transition: all 0.2s ease;
}
.legend__element-type__metals__alkali-metal:hover, .legend__element-type__metals__alkali-earth-metal:hover, .legend__element-type__metals__transition-metal:hover, .legend__element-type__metals__post-transition-metal:hover, .legend__element-type__nonmetals__alkali-metal:hover, .legend__element-type__nonmetals__alkali-earth-metal:hover, .legend__element-type__nonmetals__transition-metal:hover, .legend__element-type__nonmetals__post-transition-metal:hover {
transform: scale(1.075);
}
.legend__element-type__metals__alkali-metal span, .legend__element-type__metals__alkali-earth-metal span, .legend__element-type__metals__transition-metal span, .legend__element-type__metals__post-transition-metal span, .legend__element-type__nonmetals__alkali-metal span, .legend__element-type__nonmetals__alkali-earth-metal span, .legend__element-type__nonmetals__transition-metal span, .legend__element-type__nonmetals__post-transition-metal span {
float: left;
font-size: 0.85rem;
width: 100px;
}
.legend__element-type__metals__lanthanoid-actinoid, .legend__element-type__nonmetals__lanthanoid-actinoid {
align-content: space-between;
justify-content: center;
width: 105px;
margin-right: 5px;
}
.legend__element-type__metals__lanthanoid-actinoid .lanthanoid,
.legend__element-type__metals__lanthanoid-actinoid .actinoid, .legend__element-type__nonmetals__lanthanoid-actinoid .lanthanoid,
.legend__element-type__nonmetals__lanthanoid-actinoid .actinoid {
justify-content: center;
align-items: center;
width: 100%;
height: 48%;
border: 2px solid;
transition: all 0.2s ease;
}
.legend__element-type__metals__lanthanoid-actinoid .lanthanoid span,
.legend__element-type__metals__lanthanoid-actinoid .actinoid span, .legend__element-type__nonmetals__lanthanoid-actinoid .lanthanoid span,
.legend__element-type__nonmetals__lanthanoid-actinoid .actinoid span {
font-size: 0.85rem;
}
.legend__element-type__metals__lanthanoid-actinoid .lanthanoid:hover,
.legend__element-type__metals__lanthanoid-actinoid .actinoid:hover, .legend__element-type__nonmetals__lanthanoid-actinoid .lanthanoid:hover,
.legend__element-type__nonmetals__lanthanoid-actinoid .actinoid:hover {
transform: scale(1.075);
}
.legend__element-type__metals__lanthanoid-actinoid .lanthanoid, .legend__element-type__nonmetals__lanthanoid-actinoid .lanthanoid {
border-color: #f4c3a9;
color: #f4c3a9;
}
.legend__element-type__metals__lanthanoid-actinoid .actinoid, .legend__element-type__nonmetals__lanthanoid-actinoid .actinoid {
border-color: #ea9971;
color: #ea9971;
}
.legend__element-type__metals__alkali-metal, .legend__element-type__nonmetals__alkali-metal {
border-color: #e74d3c;
color: #e74d3c;
}
.legend__element-type__metals__alkali-metal span, .legend__element-type__nonmetals__alkali-metal span {
transform: rotate(90deg) translate(0, 280%);
}
.legend__element-type__metals__alkali-earth-metal, .legend__element-type__nonmetals__alkali-earth-metal {
border-color: #e77e23;
color: #e77e23;
}
.legend__element-type__metals__alkali-earth-metal span, .legend__element-type__nonmetals__alkali-earth-metal span {
transform: rotate(90deg) translate(0, 140%);
}
.legend__element-type__metals__transition-metal, .legend__element-type__nonmetals__transition-metal {
border-color: #f1c40e;
color: #f1c40e;
}
.legend__element-type__metals__transition-metal span, .legend__element-type__nonmetals__transition-metal span {
transform: rotate(90deg) translate(0, 138%);
}
.legend__element-type__metals__post-transition-metal, .legend__element-type__nonmetals__post-transition-metal {
margin-right: 0;
border-color: #1abc9c;
color: #1abc9c;
}
.legend__element-type__metals__post-transition-metal span, .legend__element-type__nonmetals__post-transition-metal span {
transform: rotate(90deg) translate(0, 138%);
}
.legend__element-type__metals::after, .legend__element-type__nonmetals::after {
position: absolute;
top: 0;
left: 0;
right: 0;
text-align: center;
margin-top: -3px;
border-right: 2px solid white;
border-left: 2px solid white;
transform: translateY(-100%);
}
.legend__element-type__metals {
width: 245px;
}
.legend__element-type__metals::after {
content: "Metals";
}
.legend__element-type__nonmetals {
width: 70px;
}
.legend__element-type__nonmetals__other-nonmetal, .legend__element-type__nonmetals__noble-gas {
width: 32.5px;
text-align: center;
transition: all 0.2s ease;
}
.legend__element-type__nonmetals__other-nonmetal span, .legend__element-type__nonmetals__noble-gas span {
transform: rotate(90deg) translate(35%, 138%);
}
.legend__element-type__nonmetals__other-nonmetal:hover, .legend__element-type__nonmetals__noble-gas:hover {
transform: scale(1.075);
}
.legend__element-type__nonmetals__other-nonmetal {
margin-right: 5px;
border: 2px solid #95a6a7;
color: #95a6a7;
}
.legend__element-type__nonmetals__noble-gas {
border: 2px solid #8d43ad;
color: #8d43ad;
}
.legend__element-type__nonmetals__noble-gas span {
transform: rotate(90deg) translate(45%, 280%);
}
.legend__element-type__nonmetals span {
float: left;
font-size: 0.85rem;
width: 100px;
}
.legend__element-type__nonmetals::after {
content: "Non-metals";
}
.legend__element-type__metalloid {
height: 100%;
width: 35px;
color: #3398db;
border: 2px solid #3398db;
transition: all 0.2s ease;
}
.legend__element-type__metalloid span {
float: left;
font-size: 0.85rem;
width: 100px;
transform: rotate(90deg) translate(70%, 265%);
}
.legend__element-type__metalloid:hover {
transform: scale(1.075);
}
.legend__element-type__unknown {
width: 35px;
height: 100%;
border: 2px solid #b7950b;
color: #b7950b;
transition: all 0.2s ease;
}
.legend__element-type__unknown span {
float: left;
font-size: 0.85rem;
width: 100px;
transform: rotate(90deg) translate(0, 55%);
}
.legend__element-type__unknown:hover {
transform: scale(1.075);
}
.legend__element-state {
justify-content: space-between;
align-content: space-between;
width: 125px;
height: 100%;
}
.legend__element-state__gas, .legend__element-state__liquid, .legend__element-state__solid, .legend__element-state__unknown {
justify-content: center;
align-items: center;
width: 58.75px;
height: 58.75px;
border: 2px white;
transition: all 0.2s ease;
}
.legend__element-state__gas span, .legend__element-state__liquid span, .legend__element-state__solid span, .legend__element-state__unknown span {
flex-basis: 100%;
text-align: center;
font-size: 0.85rem;
}
.legend__element-state__gas:hover, .legend__element-state__liquid:hover, .legend__element-state__solid:hover, .legend__element-state__unknown:hover {
transform: scale(1.075);
}
.legend__element-state__gas {
border-style: dashed;
}
.legend__element-state__liquid {
border-style: solid;
}
.legend__element-state__solid {
border: double;
}
.legend__element-state__unknown {
border-style: dotted;
}
.temperature {
align-items: center;
justify-content: space-between;
grid-area: 3/3/3/13;
margin-left: 46.6666666667px;
}
.temperature__inputs {
align-items: center;
}
.temperature__inputs__slider {
position: relative;
width: 397.5px;
height: 3.3333333333px;
border-radius: 5px;
background-color: white;
padding: 0;
margin: 0 10px 0 0;
-webkit-appearance: none;
-moz-appearance: none;
appearance: none;
outline: none;
}
.temperature__inputs__slider::after {
content: "Drag and drop to modify temperature and element's state";
position: absolute;
top: -1.5rem;
left: 0;
right: 0;
text-align: center;
font-family: "Josefin Sans", "Roboto", sans-serif;
color: white;
}
.temperature__inputs__slider::-webkit-slider-thumb {
width: 20px;
height: 20px;
-webkit-appearance: none;
appearance: none;
border-radius: 50%;
background-color: #6f7376;
cursor: pointer;
-webkit-transition: all 0.2s ease-in-out;
transition: all 0.2s ease-in-out;
}
.temperature__inputs__slider::-webkit-slider-thumb::after {
background-color: #6f7376;
}
.temperature__inputs__slider::-webkit-slider-thumb:hover {
background-color: #a3a6a8;
}
.temperature__inputs__slider::-webkit-slider-thumb:active::-webkit-slider-thumb {
background-color: #898c90;
}
.temperature__inputs__slider::-moz-range-thumb {
width: 17.5px;
height: 17.5px;
-moz-appearance: none;
appearance: none;
border-radius: 50%;
background-color: #6f7376;
cursor: pointer;
-moz-transition: all 0.2s ease-in-out;
transition: all 0.2s ease-in-out;
}
.temperature__inputs__slider::-moz-range-thumb:hover {
background-color: #a3a6a8;
}
.temperature__inputs__slider::-moz-range-thumb::-moz-range-thumb {
background-color: #898c90;
}
.temperature__inputs ::-moz-range-track {
background: white;
border: 0;
}
.temperature__inputs input::-moz-focus-inner,
.temperature__inputs input::-moz-focus-outer {
border: 0;
}
.temperature__inputs__result {
position: relative;
align-items: center;
justify-content: center;
width: 65px;
height: 35px;
color: white;
border: 2px solid #6f7376;
}
.temperature__inputs__result::after {
content: "";
position: absolute;
bottom: 1px;
left: 0;
border-top: 5px solid transparent;
border-right: 7.5px solid #6f7376;
border-bottom: 5px solid transparent;
transform: translate(-100%, -100%);
}
.temperature__unit-conversion {
align-content: space-evenly;
text-align: center;
width: 105px;
height: 100%;
}
.temperature__unit-conversion p {
flex-basis: 100%;
opactity: 0.5;
}
.temperature .reset__temperature {
opacity: 0;
visibility: hidden;
justify-content: center;
align-content: center;
position: relative;
width: 105px;
height: 46.6666666667px;
overflow: hidden;
background: transparent;
margin-right: 1.85rem;
font-family: "Josefin Sans", "Roboto", sans-serif;
color: white;
border: 2px solid #6f7376;
background: none;
border-radius: 2px;
cursor: pointer;
outline: none;
z-index: 0;
transition: all 0.3s ease;
}
.temperature .reset__temperature span {
position: relative;
z-index: 100;
}
.temperature .reset__temperature.--is-visible {
opacity: 1;
visibility: visible;
}
.temperature .reset__temperature::before, .temperature .reset__temperature::after {
content: "";
position: absolute;
top: 50%;
width: 15px;
height: 15px;
background-color: #6f7376;
border-radius: 50%;
}
.temperature .reset__temperature::before {
left: -1.25rem;
transform: translate(-50%, -50%);
}
.temperature .reset__temperature::after {
right: -1.25rem;
transform: translate(50%, -50%);
}
.temperature .reset__temperature:hover {
-webkit-animation: focus 0.001s 0.8s forwards;
animation: focus 0.001s 0.8s forwards;
}
.temperature .reset__temperature:hover::before {
-webkit-animation: criss-cross-left 1s both alternate, lighten-background 0.001s 0.8s forwards;
animation: criss-cross-left 1s both alternate, lighten-background 0.001s 0.8s forwards;
}
.temperature .reset__temperature:hover::after {
-webkit-animation: criss-cross-right 1s both alternate, lighten-background 0.001s 0.8s forwards;
animation: criss-cross-right 1s both alternate, lighten-background 0.001s 0.8s forwards;
}
.temperature .reset__temperature:active {
transform: scale(0.95);
}
.element {
position: relative;
width: 70px;
height: 70px;
justify-content: center;
align-items: center;
border: 2px solid;
cursor: pointer;
font-size: 0.75rem;
transition: all 0.2s ease;
}
.element[data-element-type=other-nonmetal] {
color: #95a6a7;
border-color: #95a6a7;
}
.element[data-element-type=alkali-metal] {
color: #e74d3c;
border-color: #e74d3c;
}
.element[data-element-type=alkali-earth-metal] {
color: #e77e23;
border-color: #e77e23;
}
.element[data-element-type=transition-metal] {
color: #f1c40e;
border-color: #f1c40e;
}
.element[data-element-type=Lanthanoid] {
color: #f4c3a9;
border-color: #f4c3a9;
}
.element[data-element-type=Actinoid] {
color: #ea9971;
border-color: #ea9971;
}
.element[data-element-type=post-transition-metal] {
color: #1abc9c;
border-color: #1abc9c;
}
.element[data-element-type=metalloid] {
color: #3398db;
border-color: #3398db;
}
.element[data-element-type=noble-gas] {
color: #8d43ad;
border-color: #8d43ad;
}
.element[data-element-type=unknown] {
color: #b7950b;
border-color: #b7950b;
}
.element[data-element-state=gas] {
border-style: dashed;
}
.element[data-element-state=liquid] {
border-style: solid;
}
.element[data-element-state=solid] {
border: double;
}
.element[data-element-state=unknown] {
border-style: dotted;
}
.element[data-element-name=Hydrogen] {
grid-area: 1/1;
margin-top: 1px;
}
.element[data-element-name=Lithium] {
grid-area: 2/1;
}
.element[data-element-name=Sodium] {
grid-area: 3/1;
}
.element[data-element-name=Potassium] {
grid-area: 4/1;
}
.element[data-element-name=Rubidium] {
grid-area: 5/1;
}
.element[data-element-name=Caesium] {
grid-area: 6/1;
}
.element[data-element-name=Francium] {
grid-area: 7/1;
}
.element[data-element-name=Beryllium] {
grid-area: 2/2;
}
.element[data-element-name=Magnesium] {
grid-area: 3/2;
}
.element[data-element-name=Calcium] {
grid-area: 4/2;
}
.element[data-element-name=Stronium] {
grid-area: 5/2;
}
.element[data-element-name=Barium] {
grid-area: 6/2;
}
.element[data-element-name=Radium] {
grid-area: 7/2;
}
.element[data-element-name=Scandium] {
grid-area: 4/3;
}
.element[data-element-name=Yttrium] {
grid-area: 5/3;
}
.element[data-element-name=Lanthanoids] {
grid-area: 6/3;
}
.element[data-element-name=Actinoids] {
grid-area: 7/3;
}
.element[data-element-name=Titanium] {
grid-area: 4/4;
}
.element[data-element-name=Zirconium] {
grid-area: 5/4;
}
.element[data-element-name=Hafnium] {
grid-area: 6/4;
}
.element[data-element-name=Rutherfordium] {
grid-area: 7/4;
}
.element[data-element-name=Vanadium] {
grid-area: 4/5;
}
.element[data-element-name=Niobium] {
grid-area: 5/5;
}
.element[data-element-name=Tantalum] {
grid-area: 6/5;
}
.element[data-element-name=Dubnium] {
grid-area: 7/5;
}
.element[data-element-name=Chromium] {
grid-area: 4/6;
}
.element[data-element-name=Molybdenum] {
grid-area: 5/6;
}
.element[data-element-name=Tungsten] {
grid-area: 6/6;
}
.element[data-element-name=Seaborgium] {
grid-area: 7/6;
}
.element[data-element-name=Manganese] {
grid-area: 4/7;
}
.element[data-element-name=Technetium] {
grid-area: 5/7;
}
.element[data-element-name=Rhenium] {
grid-area: 6/7;
}
.element[data-element-name=Bohrium] {
grid-area: 7/7;
}
.element[data-element-name=Iron] {
grid-area: 4/8;
}
.element[data-element-name=Ruthenium] {
grid-area: 5/8;
}
.element[data-element-name=Osmium] {
grid-area: 6/8;
}
.element[data-element-name=Hassium] {
grid-area: 7/8;
}
.element[data-element-name=Cobalt] {
grid-area: 4/9;
}
.element[data-element-name=Rhodium] {
grid-area: 5/9;
}
.element[data-element-name=Iridium] {
grid-area: 6/9;
}
.element[data-element-name=Meitnerium] {
grid-area: 7/9;
}
.element[data-element-name=Nickel] {
grid-area: 4/10;
}
.element[data-element-name=Palladium] {
grid-area: 5/10;
}
.element[data-element-name=Platinum] {
grid-area: 6/10;
}
.element[data-element-name=Darmstadtium] {
grid-area: 7/10;
}
.element[data-element-name=Copper] {
grid-area: 4/11;
}
.element[data-element-name=Silver] {
grid-area: 5/11;
}
.element[data-element-name=Gold] {
grid-area: 6/11;
}
.element[data-element-name=Roentgenium] {
grid-area: 7/11;
}
.element[data-element-name=Zinc] {
grid-area: 4/12;
}
.element[data-element-name=Cadmium] {
grid-area: 5/12;
}
.element[data-element-name=Mercury] {
grid-area: 6/12;
}
.element[data-element-name=Copernicium] {
grid-area: 7/12;
}
.element[data-element-name=Boron] {
grid-area: 2/13;
}
.element[data-element-name=Aluminium] {
grid-area: 3/13;
}
.element[data-element-name=Galium] {
grid-area: 4/13;
}
.element[data-element-name=Indium] {
grid-area: 5/13;
}
.element[data-element-name=Thalium] {
grid-area: 6/13;
}
.element[data-element-name=Nihonium] {
grid-area: 7/13;
}
.element[data-element-name=Carbon] {
grid-area: 2/14;
}
.element[data-element-name=Silicon] {
grid-area: 3/14;
}
.element[data-element-name=Germanium] {
grid-area: 4/14;
}
.element[data-element-name=Tin] {
grid-area: 5/14;
}
.element[data-element-name=Lead] {
grid-area: 6/14;
}
.element[data-element-name=Flerovium] {
grid-area: 7/14;
}
.element[data-element-name=Nitrogen] {
grid-area: 2/15;
}
.element[data-element-name=Phosphorus] {
grid-area: 3/15;
}
.element[data-element-name=Arsenic] {
grid-area: 4/15;
}
.element[data-element-name=Antimony] {
grid-area: 5/15;
}
.element[data-element-name=Bismuth] {
grid-area: 6/15;
}
.element[data-element-name=Moscovium] {
grid-area: 7/15;
}
.element[data-element-name=Oxygen] {
grid-area: 2/16;
}
.element[data-element-name=Sulfur] {
grid-area: 3/16;
}
.element[data-element-name=Selenium] {
grid-area: 4/16;
}
.element[data-element-name=Tellurium] {
grid-area: 5/16;
}
.element[data-element-name=Polonium] {
grid-area: 6/16;
}
.element[data-element-name=Livemorium] {
grid-area: 7/16;
}
.element[data-element-name=Fluorine] {
grid-area: 2/17;
}
.element[data-element-name=Chlorine] {
grid-area: 3/17;
}
.element[data-element-name=Bromine] {
grid-area: 4/17;
}
.element[data-element-name=Iodine] {
grid-area: 5/17;
}
.element[data-element-name=Astatine] {
grid-area: 6/17;
}
.element[data-element-name=Tennessine] {
grid-area: 7/17;
}
.element[data-element-name=Helium] {
grid-area: 1/18;
}
.element[data-element-name=Neon] {
grid-area: 2/18;
}
.element[data-element-name=Argon] {
grid-area: 3/18;
}
.element[data-element-name=Krypton] {
grid-area: 4/18;
}
.element[data-element-name=Xenon] {
grid-area: 5/18;
}
.element[data-element-name=Radon] {
grid-area: 6/18;
}
.element[data-element-name=Oganesson] {
grid-area: 7/18;
}
.element[data-element-name=Lanthanum] {
grid-area: 9/3;
}
.element[data-element-name=Cerium] {
grid-area: 9/4;
}
.element[data-element-name=Praseodymium] {
grid-area: 9/5;
}
.element[data-element-name=Neodymium] {
grid-area: 9/6;
}
.element[data-element-name=Promethium] {
grid-area: 9/7;
}
.element[data-element-name=Samarium] {
grid-area: 9/8;
}
.element[data-element-name=Europium] {
grid-area: 9/9;
}
.element[data-element-name=Gadolinium] {
grid-area: 9/10;
}
.element[data-element-name=Terbium] {
grid-area: 9/11;
}
.element[data-element-name=Dysprosium] {
grid-area: 9/12;
}
.element[data-element-name=Holmium] {
grid-area: 9/13;
}
.element[data-element-name=Erbium] {
grid-area: 9/14;
}
.element[data-element-name=Thulium] {
grid-area: 9/15;
}
.element[data-element-name=Ytterbium] {
grid-area: 9/16;
}
.element[data-element-name=Lutetium] {
grid-area: 9/17;
}
.element[data-element-name=Actinium] {
grid-area: 10/3;
}
.element[data-element-name=Thorium] {
grid-area: 10/4;
}
.element[data-element-name=Protactinium] {
grid-area: 10/5;
}
.element[data-element-name=Uranium] {
grid-area: 10/6;
}
.element[data-element-name=Neptunium] {
grid-area: 10/7;
}
.element[data-element-name=Plutonium] {
grid-area: 10/8;
}
.element[data-element-name=Americium] {
grid-area: 10/9;
}
.element[data-element-name=Curium] {
grid-area: 10/10;
}
.element[data-element-name=Berkelium] {
grid-area: 10/11;
}
.element[data-element-name=Californium] {
grid-area: 10/12;
}
.element[data-element-name=Einsteinium] {
grid-area: 10/13;
}
.element[data-element-name=Fermium] {
grid-area: 10/14;
}
.element[data-element-name=Mendelevium] {
grid-area: 10/15;
}
.element[data-element-name=Nobelium] {
grid-area: 10/16;
}
.element[data-element-name=Lawrencium] {
grid-area: 10/17;
}
.element .tooltip {
position: absolute;
padding: 0.25rem;
text-align: center;
background-color: #191a1a;
color: white;
border-radius: 1px;
visibility: hidden;
z-index: 100;
transition: visibility 0.15s cubic-bezier(0.23, 1, 0.32, 1);
}
.element .tooltip.--is-hidden {
display: none;
}
.element .tooltip.right-tooltip {
left: 100%;
margin-left: 0.75rem;
}
.element .tooltip.right-tooltip::after {
content: "";
position: absolute;
top: 50%;
right: 100%;
border-top: 5px solid transparent;
border-right: 7.5px solid #191a1a;
border-bottom: 5px solid transparent;
transform: translateY(-50%);
}
.element .tooltip.left-tooltip {
right: 100%;
margin-right: 0.75rem;
}
.element .tooltip.left-tooltip::after {
content: "";
position: absolute;
top: 50%;
left: 100%;
border-top: 5px solid transparent;
border-left: 7.5px solid #191a1a;
border-bottom: 5px solid transparent;
transform: translateY(-50%);
}
.element .tooltip.top-tooltip {
top: -0.75rem;
transform: translateY(-100%);
}
.element .tooltip.top-tooltip::after {
content: "";
position: absolute;
bottom: -0.9rem;
left: 50%;
border-top: 7.5px solid #191a1a;
border-right: 5px solid transparent;
border-left: 5px solid transparent;
transform: translate(-50%, -100%);
}
.element .tooltip.bottom-tooltip {
bottom: -0.75rem;
transform: translateY(100%);
}
.element .tooltip.bottom-tooltip::after {
content: "";
position: absolute;
bottom: 0.75rem;
left: 50%;
border-bottom: 7.5px solid #191a1a;
border-right: 5px solid transparent;
border-left: 5px solid transparent;
transform: translate(-50%, -100%);
}
.element:hover:not([data-element-name=Lanthanoids]):not([data-element-name=Actinoids]), .element.--is-active {
box-shadow: 0 0 1rem #888686;
transform: scale(1.075);
}
.element.--is-triggered {
z-index: 10;
}
.element:hover[data-element-name=Lanthanoids], .element:hover[data-element-name=Actinoids] {
transform: scale(1.075);
}
.element:hover .tooltip {
visibility: visible;
}
.modal {
align-items: center;
justify-content: center;
position: fixed;
top: 0;
left: 0;
right: 0;
bottom: 0;
visibility: hidden;
opacity: 0;
background-color: rgba(59, 58, 58, 0.975);
z-index: 1000;
will-change: visibility, opacity;
transition: all 0.5s cubic-bezier(0.23, 1, 0.32, 1);
}
.modal__dialog {
position: relative;
max-width: 750px;
width: 100%;
max-height: 90%;
height: 100%;
padding: 1.2rem;
border: none;
background: none;
}
.modal__content {
position: absolute;
top: 0;
left: 0;
width: 100%;
height: 100%;
padding: 1.5rem;
opacity: 0;
border: 3px solid transparent;
transition: all 0.25s cubic-bezier(0.23, 1, 0.32, 1);
}
.modal__content.--is-visible {
opacity: 1;
border: 3px solid transparent;
color: white;
}
.modal__content.--is-visible::before, .modal__content.--is-visible::after {
transform: scale(1);
}
.modal__content .atom {
position: absolute;
top: 80px;
left: 0;
right: 0;
-webkit-animation: up-and-down 7s ease infinite;
animation: up-and-down 7s ease infinite;
}
.modal__content .atom__core {
position: absolute;
top: 50%;
left: 50%;
width: 1.5rem;
height: 1.5rem;
border-radius: 50%;
background-color: #7c7979;
z-index: 0;
}
.modal__content .atom::before, .modal__content .atom::after {
content: "";
position: absolute;
top: 50%;
left: 50%;
margin: -65px -65px 0;
width: 150px;
height: 150px;
border-radius: 50%;
border: 4px solid #7c7979;
}
.modal__content .atom::before {
z-index: 1;
-webkit-animation: clockwise-rotation-2 2s linear infinite;
animation: clockwise-rotation-2 2s linear infinite;
}
.modal__content .atom::after {
z-index: 2;
-webkit-animation: clockwise-rotation-1 2s linear infinite;
animation: clockwise-rotation-1 2s linear infinite;
}
.modal__content::before, .modal__content::after {
content: "";
position: absolute;
top: 0;
left: 0;
width: 100%;
height: 100%;
z-index: 3;
transform: scale(0);
transition: all 0.7s cubic-bezier(0.23, 1, 0.32, 1);
}
.modal__content::before {
border-bottom: 3px solid #7c7979;
border-left: 3px solid #7c7979;
transform-origin: 0 100%;
}
.modal__content::after {
border-top: 3px solid #7c7979;
border-right: 3px solid #7c7979;
transform-origin: 100% 0%;
}
.modal__content__properties {
display: grid;
gap: 0 0.5rem;
grid-template: repeat(auto-fill, minmax(70px, 1fr))/repeat(9, 70px);
height: 100%;
}
.modal__close {
position: absolute;
top: 1rem;
right: 1rem;
width: 1.75rem;
height: 1.75rem;
padding: 0.15rem;
background: transparent;
cursor: pointer;
z-index: 100;
transition: all 0.5s cubic-bezier(0.23, 1, 0.32, 1);
}
.modal__close svg {
width: 1.75rem;
height: 1.75rem;
border: 2px solid #7c7979;
fill: #7c7979;
pointer-events: none;
transition: all 0.5s cubic-bezier(0.23, 1, 0.32, 1);
}
.modal__close:hover svg {
background: #7c7979;
fill: #3b3a3a;
}
.modal.--modal__background {
background: #888686;
}
.modal.--is-visible {
visibility: visible;
opacity: 1;
}
.modal__content__properties .content {
text-transform: math-auto;
}
.modal__content__properties .element-property {
text-align: center;
gap: 0.25rem;
}
.modal__content__properties .element-property .title {
border-bottom: 1px solid #7c7979;
margin: 0 1rem;
}
.modal__content__properties .element-name {
grid-row: 3;
grid-column: 1/3;
}
.modal__content__properties .element-symbol {
grid-row: 3;
grid-column: 3/5;
}
.modal__content__properties .element-atomic-number {
grid-row: 3;
grid-column: 5/7;
}
.modal__content__properties .element-atomic-weight {
grid-row: 3;
grid-column: 7/10;
}
.modal__content__properties .element-group {
grid-row: 4;
grid-column: 1/2;
}
.modal__content__properties .element-period {
grid-row: 4;
grid-column: 2/3;
}
.modal__content__properties .element-category {
grid-row: 4;
grid-column: 3/8;
}
.modal__content__properties .element-block {
grid-row: 4;
grid-column: 8/10;
}
.modal__content__properties .element-electron-configuration {
grid-row: 5;
grid-column: 1/4;
}
.modal__content__properties .element-electron-per-shell {
grid-row: 5;
grid-column: 4/7;
}
.modal__content__properties .element-phase-at-STP {
grid-row: 5;
grid-column: 7/10;
}
.modal__content__properties .element-melting-point {
grid-row: 6;
grid-column: 1/3;
}
.modal__content__properties .element-boiling-point {
grid-row: 6;
grid-column: 3/6;
}
.modal__content__properties .element-oxidation-states {
grid-row: 6;
grid-column: 6/10;
}
.modal__content__properties .element-electronegativity {
grid-row: 7;
grid-column: 1/3;
}
.modal__content__properties .element-ionization-energy {
grid-row: 7;
grid-column: 3/6;
}
.modal__content__properties .element-covalent-radius {
grid-row: 7;
grid-column: 6/10;
}
.modal__content__properties .element-van-der-waals-radius {
grid-row: 8;
grid-column: 1/5;
}
.modal__content__properties .element-crystal-structure {
grid-row: 8;
grid-column: 5/10;
}
.modal__content__properties .element-thermal-conductivity {
grid-row: 9;
grid-column: 1/4;
}
.modal__content__properties .element-magnetic-order {
grid-row: 9;
grid-column: 4/7;
}
.modal__content__properties .element-magnetic-susceptibility {
grid-row: 9;
grid-column: 7/10;
}
.modal__content__properties .element-miscellaneous {
grid-row: 10;
grid-column: 1/11;
}
@-webkit-keyframes criss-cross-left {
0% {
left: -1.5rem;
}
50% {
left: 50%;
width: 15px;
height: 15px;
}
100% {
left: 50%;
width: 150%;
height: 150%;
}
}
@keyframes criss-cross-left {
0% {
left: -1.5rem;
}
50% {
left: 50%;
width: 15px;
height: 15px;
}
100% {
left: 50%;
width: 150%;
height: 150%;
}
}
@-webkit-keyframes criss-cross-right {
0% {
right: -1.5rem;
}
50% {
right: 50%;
width: 15px;
height: 15px;
}
100% {
right: 50%;
width: 150%;
height: 150%;
}
}
@keyframes criss-cross-right {
0% {
right: -1.5rem;
}
50% {
right: 50%;
width: 15px;
height: 15px;
}
100% {
right: 50%;
width: 150%;
height: 150%;
}
}
@-webkit-keyframes focus {
to {
border: 2px solid #3e4042;
color: #3e4042;
font-weight: 600;
box-shadow: 0 0 1rem #888686;
}
}
@keyframes focus {
to {
border: 2px solid #3e4042;
color: #3e4042;
font-weight: 600;
box-shadow: 0 0 1rem #888686;
}
}
@-webkit-keyframes lighten-background {
to {
background-color: #6f7376;
}
}
@keyframes lighten-background {
to {
background-color: #6f7376;
}
}
@-webkit-keyframes clockwise-rotation-1 {
from {
transform: perspective(1000px) rotate3d(1, 1, 1, 0deg);
}
to {
transform: perspective(1000px) rotate3d(1, 1, 1, 360deg);
}
}
@keyframes clockwise-rotation-1 {
from {
transform: perspective(1000px) rotate3d(1, 1, 1, 0deg);
}
to {
transform: perspective(1000px) rotate3d(1, 1, 1, 360deg);
}
}
@-webkit-keyframes clockwise-rotation-2 {
from {
transform: perspective(1000px) rotate3d(1, -1, 1, 0deg);
}
to {
transform: perspective(1000px) rotate3d(1, -1, 1, 360deg);
}
}
@keyframes clockwise-rotation-2 {
from {
transform: perspective(1000px) rotate3d(1, -1, 1, 0deg);
}
to {
transform: perspective(1000px) rotate3d(1, -1, 1, 360deg);
}
}
@-webkit-keyframes up-and-down {
0% {
margin-top: -1rem;
}
50% {
margin-top: 1rem;
}
100% {
margin-top: -1rem;
}
}
@keyframes up-and-down {
0% {
margin-top: -1rem;
}
50% {
margin-top: 1rem;
}
100% {
margin-top: -1rem;
}
}
Add JavaScript Functionality
Finally, integrate this JavaScript code to bring interactivity to your periodic table. This script manages the temperature slider, highlights elements dynamically, and presents detailed information in a modal window when an element is clicked.
console.clear();
const DELAY = 5,
STP = 273,
ELEMENT_MODAL_DATA = {
Hydrogen: {
name:
"<span class='title'>Element:</span> <span class='content'>Hydrogen</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>H</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>1</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>1.008 (conventional)</span>",
group: "<span class='title'>Group:</span> <span class='content'>1</span>",
period:
"<span class='title'>Period:</span> <span class='content'>1</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Reactive non-metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>1s<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Gas</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>13.99 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>20.271 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>-1, +1 (an amphoteric oxide)</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.20</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1312.0 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>31±5 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>120 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.1805 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−3.98·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered in 1766 by Henry Cavendish (named by Antoine Lavoisier in 1783)</span>" },
Helium: {
name:
"<span class='title'>Element:</span> <span class='content'>Helium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>He</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>2</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>4.0026</span>",
group:
"<span class='title'>Group:</span> <span class='content'>18</span>",
period:
"<span class='title'>Period:</span> <span class='content'>1</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Noble gas</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>1s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Gas</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>0.95 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>4.22 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>0</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>—</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 2372.3 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>28 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>140 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.1513 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>—</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered in 1868 by Pierre Janssen (independently by Norman Lockyer)</span>" },
Lithium: {
name:
"<span class='title'>Element:</span> <span class='content'>Lithium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Li</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>3</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>6.94</span>",
group: "<span class='title'>Group:</span> <span class='content'>1</span>",
period:
"<span class='title'>Period:</span> <span class='content'>2</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Alkali metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[He] 2s<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>453.65 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1603 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.98</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 520.2 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>128 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>182 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>84.8 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+13.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered in 1817 by Johan August Arfwedson</span>" },
Beryllium: {
name:
"<span class='title'>Element:</span> <span class='content'>Beryllium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Be</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>4</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>9.0122</span>",
group: "<span class='title'>Group:</span> <span class='content'>2</span>",
period:
"<span class='title'>Period:</span> <span class='content'>2</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Alkaline earth metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[He] 2s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1560.15 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2742 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.57</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 899.5 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>96 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>153 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>190 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+4.9·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered in 1798 by Louis Nicolas Vauquelin</span>" },
Boron: {
name:
"<span class='title'>Element:</span> <span class='content'>Boron</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>B</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>5</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>10.81</span>",
group:
"<span class='title'>Group:</span> <span class='content'>13</span>",
period:
"<span class='title'>Period:</span> <span class='content'>2</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Metalloid</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[He] 2s<sup>2</sup> 2p<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 3</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2349 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>4200 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.04</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 800.6 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>84 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>192 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>rhombohedral</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>27.4 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−5.2·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered in ancient times, isolated in 1808 by Sir Humphry Davy and Joseph Louis Gay-Lussac</span>" },
Carbon: {
name:
"<span class='title'>Element:</span> <span class='content'>Carbon</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>C</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>6</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>12.011</span>",
group:
"<span class='title'>Group:</span> <span class='content'>14</span>",
period:
"<span class='title'>Period:</span> <span class='content'>2</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Non-metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[He] 2s<sup>2</sup> 2p<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 4</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>sublimation point: 3915 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>sublimation</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+4, +3, +2, +1, −1, −2, −3, −4</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.55</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1086.5 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>77 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>170 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>140 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−5.1·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Nitrogen: {
name:
"<span class='title'>Element:</span> <span class='content'>Nitrogen</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>N</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>7</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>14.007</span>",
group:
"<span class='title'>Group:</span> <span class='content'>15</span>",
period:
"<span class='title'>Period:</span> <span class='content'>2</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Non-metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[He] 2s<sup>2</sup> 2p<sup>3</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 5</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Gas</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>63.15 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>77.36 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+5, +4, +3, +2, +1, −1, −2, −3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>3.04</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1402.3 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>75 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>155 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.02583 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−3.8·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered in 1772 by Daniel Rutherford</span>" },
Oxygen: {
name:
"<span class='title'>Element:</span> <span class='content'>Oxygen</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>O</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>8</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>15.999</span>",
group:
"<span class='title'>Group:</span> <span class='content'>16</span>",
period:
"<span class='title'>Period:</span> <span class='content'>2</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Non-metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[He] 2s<sup>2</sup> 2p<sup>4</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 6</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Gas</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>54.36 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>90.20 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>−2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>3.44</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1313.9 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>73 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>152 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.02583 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−4.3·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Fluorine: {
name:
"<span class='title'>Element:</span> <span class='content'>Fluorine</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>F</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>9</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>18.998</span>",
group:
"<span class='title'>Group:</span> <span class='content'>17</span>",
period:
"<span class='title'>Period:</span> <span class='content'>2</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Halogen</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[He] 2s<sup>2</sup> 2p<sup>5</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 7</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Gas</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>53.48 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>85.03 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>−1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>3.98</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1681.0 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>71 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>147 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.02591 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−1.8·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Isolated by Henri Moissan in 1886</span>" },
Neon: {
name:
"<span class='title'>Element:</span> <span class='content'>Neon</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ne</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>10</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>20.180</span>",
group:
"<span class='title'>Group:</span> <span class='content'>18</span>",
period:
"<span class='title'>Period:</span> <span class='content'>2</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Noble gas</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[He] 2s<sup>2</sup> 2p<sup>6</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Gas</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>24.56 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>27.07 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>0</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>—</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 2080.7 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>69 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>154 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.049 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>—</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Sir William Ramsay and Morris Travers in 1898</span>" },
Sodium: {
name:
"<span class='title'>Element:</span> <span class='content'>Sodium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Na</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>11</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>22.990</span>",
group: "<span class='title'>Group:</span> <span class='content'>1</span>",
period:
"<span class='title'>Period:</span> <span class='content'>3</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Alkali metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ne] 3s<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>370.87 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1156 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.93</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 495.8 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>154 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>227 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>142 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>6.3·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Magnesium: {
name:
"<span class='title'>Element:</span> <span class='content'>Magnesium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Mg</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>12</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>24.305</span>",
group: "<span class='title'>Group:</span> <span class='content'>2</span>",
period:
"<span class='title'>Period:</span> <span class='content'>3</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Alkaline earth metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ne] 3s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>923 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1380 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.31</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 737.7 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>141 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>173 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>156 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>3.9·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Isolated by Sir Humphry Davy in 1808</span>" },
Aluminum: {
name:
"<span class='title'>Element:</span> <span class='content'>Aluminum</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Al</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>13</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>26.982</span>",
group:
"<span class='title'>Group:</span> <span class='content'>13</span>",
period:
"<span class='title'>Period:</span> <span class='content'>3</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Post-transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ne] 3s<sup>2</sup> 3p<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 3</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>933.47 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2792 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.61</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 577.5 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>121 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>184 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>237 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>1.5·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Silicon: {
name:
"<span class='title'>Element:</span> <span class='content'>Silicon</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Si</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>14</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>28.085</span>",
group:
"<span class='title'>Group:</span> <span class='content'>14</span>",
period:
"<span class='title'>Period:</span> <span class='content'>3</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Metalloid</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ne] 3s<sup>2</sup> 3p<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 4</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1687 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3538 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+4, +2, -4</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.90</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 786.5 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>111 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>210 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>diamond cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>149 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>nonmagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>—</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Isolated by Jöns Jacob Berzelius in 1823</span>" },
Phosphorus: {
name:
"<span class='title'>Element:</span> <span class='content'>Phosphorus</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>P</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>15</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>30.974</span>",
group:
"<span class='title'>Group:</span> <span class='content'>15</span>",
period:
"<span class='title'>Period:</span> <span class='content'>3</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Nonmetal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ne] 3s<sup>2</sup> 3p<sup>3</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 5</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>317.25 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>553.65 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+5, +3, -3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.19</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1011.8 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>107 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>180 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>simple triclinic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.236 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>—</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Hennig Brand in 1669</span>" },
Sulfur: {
name:
"<span class='title'>Element:</span> <span class='content'>Sulfur</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>S</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>16</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>32.06</span>",
group:
"<span class='title'>Group:</span> <span class='content'>16</span>",
period:
"<span class='title'>Period:</span> <span class='content'>3</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Nonmetal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ne] 3s<sup>2</sup> 3p<sup>4</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 6</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>388.36 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>717.87 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+6, +4, -2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.58</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 999.6 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>102 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>180 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>orthorhombic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.205 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>—</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Chlorine: {
name:
"<span class='title'>Element:</span> <span class='content'>Chlorine</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Cl</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>17</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>35.45</span>",
group:
"<span class='title'>Group:</span> <span class='content'>17</span>",
period:
"<span class='title'>Period:</span> <span class='content'>3</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Halogen</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ne] 3s<sup>2</sup> 3p<sup>5</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 7</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Gas</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>171.6 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>239.11 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+7, +5, +3, +1, -1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>3.16</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1251.2 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>99 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>175 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>orthorhombic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.0089 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>antiferromagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−37.2·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Carl Wilhelm Scheele in 1774</span>" },
Argon: {
name:
"<span class='title'>Element:</span> <span class='content'>Argon</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ar</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>18</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>39.948</span>",
group:
"<span class='title'>Group:</span> <span class='content'>18</span>",
period:
"<span class='title'>Period:</span> <span class='content'>3</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Noble gas</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ne] 3s<sup>2</sup> 3p<sup>6</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 8</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Gas</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>83.8 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>87.3 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>0</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>—</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1520.6 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>71 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>188 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.0177 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>—</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Lord Rayleigh and Sir William Ramsay in 1894</span>" },
Potassium: {
name:
"<span class='title'>Element:</span> <span class='content'>Potassium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>K</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>19</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>39.098</span>",
group: "<span class='title'>Group:</span> <span class='content'>1</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Alkali metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 4s<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 8, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>336.53 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1032 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.82</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 418.8 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>203 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>275 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>102.5 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>5.6·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Sir Humphry Davy in 1807</span>" },
Calcium: {
name:
"<span class='title'>Element:</span> <span class='content'>Calcium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ca</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>20</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>40.078</span>",
group: "<span class='title'>Group:</span> <span class='content'>2</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Alkaline earth metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 4s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1115 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1757 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.00</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 589.8 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>176 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>231 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>201 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>—</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Sir Humphry Davy in 1808</span>" },
Scandium: {
name:
"<span class='title'>Element:</span> <span class='content'>Scandium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Sc</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>21</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>44.956</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>1</sup> 4s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 9, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1814 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3109 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.36</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 633.1 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>170 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>—</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>15.8 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>—</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Lars Fredrik Nilson in 1879</span>" },
Titanium: {
name:
"<span class='title'>Element:</span> <span class='content'>Titanium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ti</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>22</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>47.867</span>",
group: "<span class='title'>Group:</span> <span class='content'>4</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>2</sup> 4s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 10, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1941 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3560 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+4, +3, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.54</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 658.8 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>160 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>187 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>22.0 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>—</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by William Gregor in 1791</span>" },
Vanadium: {
name:
"<span class='title'>Element:</span> <span class='content'>Vanadium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>V</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>23</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>50.942</span>",
group: "<span class='title'>Group:</span> <span class='content'>5</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>3</sup> 4s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 11, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2183 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3680 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+5, +4, +3, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.63</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 650.9 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>153 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>—</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>30.7 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>antiferromagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>—</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Andrés Manuel del Río in 1801</span>" },
Chromium: {
name:
"<span class='title'>Element:</span> <span class='content'>Chromium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Cr</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>24</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>51.996</span>",
group: "<span class='title'>Group:</span> <span class='content'>6</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>5</sup> 4s<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 13, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2180 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2944 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+6, +3, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.66</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 652.9 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>166 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>—</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>93.9 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>antiferromagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>1.1·10−5 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Louis Nicolas Vauquelin in 1797</span>" },
Manganese: {
name:
"<span class='title'>Element:</span> <span class='content'>Manganese</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Mn</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>25</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>54.938</span>",
group: "<span class='title'>Group:</span> <span class='content'>7</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>5</sup> 4s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 13, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1519 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2334 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+7, +4, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.55</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 717.3 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>139 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>—</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>7.8 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>2.1·10−5 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Torbern Olof Bergman in 1774</span>" },
Iron: {
name:
"<span class='title'>Element:</span> <span class='content'>Iron</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Fe</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>26</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>55.845</span>",
group: "<span class='title'>Group:</span> <span class='content'>8</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>6</sup> 4s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 14, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1811 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3134 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.83</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 762.5 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>132 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>194 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>80.4 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>ferromagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>1.7·10−5 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Cobalt: {
name:
"<span class='title'>Element:</span> <span class='content'>Cobalt</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Co</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>27</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>58.933</span>",
group: "<span class='title'>Group:</span> <span class='content'>9</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>7</sup> 4s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 15, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1768 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3200 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.88</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 760.4 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>126 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>—</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>100 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>ferromagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>2.3·10−5 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Georg Brandt in 1735</span>" },
Nickel: {
name:
"<span class='title'>Element:</span> <span class='content'>Nickel</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ni</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>28</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>58.693</span>",
group:
"<span class='title'>Group:</span> <span class='content'>10</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>8</sup> 4s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 16, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1728 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3186 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.91</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 737.1 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>121 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>—</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>90.9 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>ferromagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>5.7·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Copper: {
name:
"<span class='title'>Element:</span> <span class='content'>Copper</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Cu</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>29</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>63.546</span>",
group:
"<span class='title'>Group:</span> <span class='content'>11</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>9</sup> 4s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1357.8 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2835 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +1 (a mild oxidizing agent)</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.9</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 745.5 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>138 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>140 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>398 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−5.8·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Zinc: {
name:
"<span class='title'>Element:</span> <span class='content'>Zinc</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Zn</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>30</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>65.38</span>",
group:
"<span class='title'>Group:</span> <span class='content'>12</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>10</sup> 4s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>692.68 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1180 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.65</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 906.4 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>131 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>139 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>116 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−1.2·10−5 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Gallium: {
name:
"<span class='title'>Element:</span> <span class='content'>Gallium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ga</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>31</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>69.723</span>",
group:
"<span class='title'>Group:</span> <span class='content'>13</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Post-transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>10</sup> 4s<sup>2</sup> 4p<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 3</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>302.9146 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2477 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.81</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 578.8 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>122 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>187 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>orthorhombic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>29.6 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−5.2·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Lecoq de Boisbaudran in 1875</span>" },
Germanium: {
name:
"<span class='title'>Element:</span> <span class='content'>Germanium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ge</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>32</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>72.630</span>",
group:
"<span class='title'>Group:</span> <span class='content'>14</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Metalloid</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>10</sup> 4s<sup>2</sup> 4p<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 4</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1211.4 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3106 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+4, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.01</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 762.0 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>122 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>211 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>diamond cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>60.2 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−1.4·10−5 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Clemens Winkler in 1886</span>" },
Arsenic: {
name:
"<span class='title'>Element:</span> <span class='content'>Arsenic</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>As</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>33</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>74.922</span>",
group:
"<span class='title'>Group:</span> <span class='content'>15</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Metalloid</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>10</sup> 4s<sup>2</sup> 4p<sup>3</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 5</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1090 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>887 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+5, +3, −3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.18</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 947.0 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>119 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>185 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>rhombohedral</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>50.2 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−2.4·10−5 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Selenium: {
name:
"<span class='title'>Element:</span> <span class='content'>Selenium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Se</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>34</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>78.971</span>",
group:
"<span class='title'>Group:</span> <span class='content'>16</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Non-metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>10</sup> 4s<sup>2</sup> 4p<sup>4</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 6</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>494 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>958 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+6, +4, −2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.55</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 941.0 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>120 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>190 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.523 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−6.7·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Jöns Jakob Berzelius in 1817</span>" },
Bromine: {
name:
"<span class='title'>Element:</span> <span class='content'>Bromine</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Br</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>35</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>79.904</span>",
group:
"<span class='title'>Group:</span> <span class='content'>17</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Halogen</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>10</sup> 4s<sup>2</sup> 4p<sup>5</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 7</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Liquid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>265.8 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>332.0 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+5, +4, +3, +1, −1, −5</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.96</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1139.9 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>120 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>185 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>orthorhombic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.122 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−1.6·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Antoine Jérôme Balard in 1826</span>" },
Krypton: {
name:
"<span class='title'>Element:</span> <span class='content'>Krypton</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Kr</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>36</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>83.798</span>",
group:
"<span class='title'>Group:</span> <span class='content'>18</span>",
period:
"<span class='title'>Period:</span> <span class='content'>4</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Noble gas</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Ar] 3d<sup>10</sup> 4s<sup>2</sup> 4p<sup>6</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 8</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Gas</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>115.78 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>119.93 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>3.00</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1350.8 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>110 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>202 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>cubic face-centered</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.00943 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>0</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Sir William Ramsay and Morris Travers in 1898</span>" },
Rubidium: {
name:
"<span class='title'>Element:</span> <span class='content'>Rubidium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Rb</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>37</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>85.468</span>",
group: "<span class='title'>Group:</span> <span class='content'>1</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Alkali metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 8, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>312.45 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>961 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.82</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 403.0 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>211 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>303 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>58.2 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+1.7·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Robert Bunsen and Gustav Kirchhoff in 1861</span>" },
Strontium: {
name:
"<span class='title'>Element:</span> <span class='content'>Strontium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Sr</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>38</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>87.62</span>",
group: "<span class='title'>Group:</span> <span class='content'>2</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Alkaline earth metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1050 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1650 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.95</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 549.5 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>195 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>249 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>35.3 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+1.2·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Adair Crawford and William Cruickshank in 1790</span>" },
Yttrium: {
name:
"<span class='title'>Element:</span> <span class='content'>Yttrium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Y</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>39</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>88.906</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>2</sup> 4d<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 9, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1799 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3609 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.22</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 600.0 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>180 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>212 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>17.2 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+1.9·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Johan Gadolin in 1794</span>" },
Zirconium: {
name:
"<span class='title'>Element:</span> <span class='content'>Zirconium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Zr</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>40</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>91.224</span>",
group: "<span class='title'>Group:</span> <span class='content'>4</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>2</sup> 4d<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 10, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2128 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>4682 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+4</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.33</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 640.1 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>155 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>22.6 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+3.2·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Martin Heinrich Klaproth in 1789</span>" },
Niobium: {
name:
"<span class='title'>Element:</span> <span class='content'>Niobium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Nb</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>41</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>92.906</span>",
group: "<span class='title'>Group:</span> <span class='content'>5</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>1</sup> 4d<sup>4</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 12, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2741 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>5017 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+5, +3, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.6</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 652.1 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>164 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>207 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>53.7 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+3.3·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Named after Niobe, the daughter of Tantalus, in Greek mythology</span>" },
Molybdenum: {
name:
"<span class='title'>Element:</span> <span class='content'>Molybdenum</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Mo</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>42</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>95.95</span>",
group: "<span class='title'>Group:</span> <span class='content'>6</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>1</sup> 4d<sup>5</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 13, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2896 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>4912 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+6, +5, +4, +3, +2, +1, -1, -2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.16</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 684.3 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>154 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>209 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>138 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+4.7·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known to the ancient Greeks, but isolated in 1781 by Peter Jacob Hjelm</span>" },
Technetium: {
name:
"<span class='title'>Element:</span> <span class='content'>Technetium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Tc</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>43</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>98</span>",
group: "<span class='title'>Group:</span> <span class='content'>7</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>1</sup> 4d<sup>6</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 13, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2430 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>4538 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+7, +6, +5, +4, +3, +2, +1, -1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.9</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 702 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>147 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>50.6 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>ferromagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+99.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Artificially produced, first isolated by Carlo Perrier and Emilio Segrè in 1937</span>" },
Ruthenium: {
name:
"<span class='title'>Element:</span> <span class='content'>Ruthenium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ru</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>44</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>101.07</span>",
group: "<span class='title'>Group:</span> <span class='content'>8</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>1</sup> 4d<sup>7</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 15, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2607 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>4423 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +4, +7, +8</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.2</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 710.2 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>146 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>120 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+8.4·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Karl Ernst Claus in 1844</span>" },
Rhodium: {
name:
"<span class='title'>Element:</span> <span class='content'>Rhodium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Rh</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>45</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>102.91</span>",
group: "<span class='title'>Group:</span> <span class='content'>9</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>1</sup> 4d<sup>8</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 16, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2237 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3968 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +2, +1, -1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.28</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 719.7 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>142 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>150 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+4.3·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by William Hyde Wollaston in 1803</span>" },
Palladium: {
name:
"<span class='title'>Element:</span> <span class='content'>Palladium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Pd</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>46</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>106.42</span>",
group:
"<span class='title'>Group:</span> <span class='content'>10</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>0</sup> 4d<sup>10</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 18, 0</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1828 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3236 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+4, +2, 0</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.2</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 804.4 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>139 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>163 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>71.8 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+5.7·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by William Hyde Wollaston in 1803</span>" },
Silver: {
name:
"<span class='title'>Element:</span> <span class='content'>Silver</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ag</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>47</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>107.87</span>",
group:
"<span class='title'>Group:</span> <span class='content'>11</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>1</sup> 4d<sup>10</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 18, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1234 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2435 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.93</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 731 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>145 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>172 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>429 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>-19.5·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Cadmium: {
name:
"<span class='title'>Element:</span> <span class='content'>Cadmium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Cd</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>48</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>112.41</span>",
group:
"<span class='title'>Group:</span> <span class='content'>12</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>2</sup> 4d<sup>10</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 18, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>594.22 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1040 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.69</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 867.8 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>148 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>158 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>96.6 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>+4.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Karl Samuel Leberecht Hermann and Friedrich Stromeyer in 1817</span>" },
Indium: {
name:
"<span class='title'>Element:</span> <span class='content'>Indium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>In</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>49</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>114.82</span>",
group:
"<span class='title'>Group:</span> <span class='content'>13</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Post-transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>2</sup> 4d<sup>10</sup> 5p<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 18, 3</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>429.75 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2345 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.78</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 558.3 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>142 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>193 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>tetragonal</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>81.8 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−76.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Ferdinand Reich and Hieronymous Theodor Richter in 1863</span>" },
Tin: {
name:
"<span class='title'>Element:</span> <span class='content'>Tin</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Sn</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>50</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>118.71</span>",
group:
"<span class='title'>Group:</span> <span class='content'>14</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Post-transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>2</sup> 4d<sup>10</sup> 5p<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 18, 4</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>505.08 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2875 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+4, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.96</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 708.6 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>141 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>217 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>tetragonal</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>66.8 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−33.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Antimony: {
name:
"<span class='title'>Element:</span> <span class='content'>Antimony</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Sb</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>51</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>121.76</span>",
group:
"<span class='title'>Group:</span> <span class='content'>15</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Metalloid</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>2</sup> 4d<sup>10</sup> 5p<sup>3</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 18, 5</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>903.78 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1860 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+5, +3, -3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.05</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 834 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>138 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>206 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>rhombohedral</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>24.6 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−61.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Tellurium: {
name:
"<span class='title'>Element:</span> <span class='content'>Tellurium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Te</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>52</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>127.60</span>",
group:
"<span class='title'>Group:</span> <span class='content'>16</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Metalloid</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>2</sup> 4d<sup>10</sup> 5p<sup>4</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 18, 6</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>722.66 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1261 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+6, +4, -2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.1</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 869 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>138 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>206 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>2.35 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−19.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Franz-Joseph Müller von Reichenstein in 1782</span>" },
Iodine: {
name:
"<span class='title'>Element:</span> <span class='content'>Iodine</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>I</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>53</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>126.90</span>",
group:
"<span class='title'>Group:</span> <span class='content'>17</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Halogen</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>2</sup> 4d<sup>10</sup> 5p<sup>5</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 18, 7</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>386.85 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>457.4 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+7, +5, +1, -1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.66</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1008.4 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>139 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>198 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>orthorhombic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.449 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−27.4·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Bernard Courtois in 1811</span>" },
Xenon: {
name:
"<span class='title'>Element:</span> <span class='content'>Xenon</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Xe</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>54</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>131.29</span>",
group:
"<span class='title'>Group:</span> <span class='content'>18</span>",
period:
"<span class='title'>Period:</span> <span class='content'>5</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Noble gas</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Kr] 5s<sup>2</sup> 4d<sup>10</sup> 5p<sup>6</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 18, 8</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Gas</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>161.4 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>165.0 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +4, +6, +8</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.60</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1170.4 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>140 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>216 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>0.00565 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>0.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Sir William Ramsay and Morris Travers in 1898</span>" },
Cesium: {
name:
"<span class='title'>Element:</span> <span class='content'>Cesium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Cs</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>55</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>132.91</span>",
group: "<span class='title'>Group:</span> <span class='content'>1</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Alkali metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 6s<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 18, 8, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>301.59 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>944 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.79</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 375.7 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>244 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>35.9 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>68.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Robert Bunsen and Gustav Kirchhoff in 1860</span>" },
Barium: {
name:
"<span class='title'>Element:</span> <span class='content'>Barium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ba</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>56</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>137.33</span>",
group: "<span class='title'>Group:</span> <span class='content'>2</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Alkaline earth metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 18, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1000 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2170 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.89</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 502.9 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>215 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>18.4 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−25.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Carl Wilhelm Scheele in 1772</span>" },
Lanthanum: {
name:
"<span class='title'>Element:</span> <span class='content'>Lanthanum</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>La</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>57</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>138.91</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 5d<sup>1</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 18, 9, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1193 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3737 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.10</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 538.1 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>207 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>13.4 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−46.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Carl Gustaf Mosander in 1839</span>" },
Cerium: {
name:
"<span class='title'>Element:</span> <span class='content'>Cerium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ce</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>58</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>140.12</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>1</sup> 5d<sup>1</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 19, 9, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1068 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3716 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+4, +3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.12</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 534.4 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>204 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>11.3 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−32.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Martin Heinrich Klaproth and Jöns Jakob Berzelius in 1803</span>" },
Praseodymium: {
name:
"<span class='title'>Element:</span> <span class='content'>Praseodymium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Pr</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>59</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>140.91</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>3</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 21, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1208 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3793 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.13</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 527.0 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>203 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>double hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>12.5 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−16.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Carl Auer von Welsbach in 1885</span>" },
Neodymium: {
name:
"<span class='title'>Element:</span> <span class='content'>Neodymium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Nd</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>60</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>144.24</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>4</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 22, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1297 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3347 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.14</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 533.1 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>201 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>double hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>16.5 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−19.5·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Carl Auer von Welsbach in 1885</span>" },
Promethium: {
name:
"<span class='title'>Element:</span> <span class='content'>Promethium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Pm</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>61</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>145</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>5</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 23, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1315 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3273 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.13</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 540 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>none</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>12.9 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−9.8·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Jacob A. Marinsky, Lawrence E. Glendenin, and Charles D. Coryell in 1945</span>" },
Samarium: {
name:
"<span class='title'>Element:</span> <span class='content'>Samarium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Sm</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>62</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>150.36</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>6</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 24, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1345 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2067 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.17</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 544.5 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>198 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>rhombohedral</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>13.3 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>antiferromagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−44.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Lecoq de Boisbaudran in 1879</span>" },
Europium: {
name:
"<span class='title'>Element:</span> <span class='content'>Europium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Eu</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>63</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>151.97</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>7</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 25, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1099 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1802 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.2</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 547.1 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>198 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>13.9 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−27.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Eugène-Anatole Demarçay in 1901</span>" },
Gadolinium: {
name:
"<span class='title'>Element:</span> <span class='content'>Gadolinium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Gd</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>64</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>157.25</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>7</sup> 5d<sup>1</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 25, 9, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1585 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3546 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.2</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 593.4 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>180 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>10.6 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−40.5·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Jean Charles Galissard de Marignac in 1880</span>" },
Terbium: {
name:
"<span class='title'>Element:</span> <span class='content'>Terbium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Tb</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>65</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>158.93</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>9</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 27, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1629 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3503 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.2</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 565.8 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>176 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>11.1 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−19.8·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Carl Gustaf Mosander in 1843</span>" },
Dysprosium: {
name:
"<span class='title'>Element:</span> <span class='content'>Dysprosium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Dy</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>66</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>162.50</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>10</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 28, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1680 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2840 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.22</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 573.0 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>170 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>10.7 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−15.8·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Paul Émile Lecoq de Boisbaudran in 1886</span>" },
Holmium: {
name:
"<span class='title'>Element:</span> <span class='content'>Holmium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ho</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>67</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>164.93</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>11</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 29, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1734 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2993 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.23</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 581.0 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>176 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>16.2 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−19.3·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Per Teodor Cleve in 1879</span>" },
Erbium: {
name:
"<span class='title'>Element:</span> <span class='content'>Erbium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Er</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>68</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>167.26</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>12</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 30, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1802 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3141 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.24</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 589.3 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>175 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>14.5 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−19.7·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Carl Gustaf Mosander in 1843</span>" },
Thulium: {
name:
"<span class='title'>Element:</span> <span class='content'>Thulium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Tm</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>69</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>168.93</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>13</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 31, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1818 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2223 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.25</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 596.7 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>172 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>16.9 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−16.6·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Per Teodor Cleve in 1879</span>" },
Ytterbium: {
name:
"<span class='title'>Element:</span> <span class='content'>Ytterbium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Yb</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>70</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>173.04</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1097 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1469 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.1</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 603.4 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>None</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>38.5 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−35.1·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Jean Charles Galissard de Marignac in 1878</span>" },
Lutetium: {
name:
"<span class='title'>Element:</span> <span class='content'>Lutetium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Lu</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>71</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>174.97</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Lanthanide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>1</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 9, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1925 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3675 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.27</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 523.5 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>None</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>16.4 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−8.6·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Carl Auer von Welsbach in 1903</span>" },
Hafnium: {
name:
"<span class='title'>Element:</span> <span class='content'>Hafnium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Hf</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>72</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>178.49</span>",
group: "<span class='title'>Group:</span> <span class='content'>4</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>2</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 10, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2506 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>4876 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +3, +4</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 658.5 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>155 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>23.0 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−17.1·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Dirk Coster and Georg von Hevesy in 1923</span>" },
Tantalum: {
name:
"<span class='title'>Element:</span> <span class='content'>Tantalum</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ta</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>73</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>180.95</span>",
group: "<span class='title'>Group:</span> <span class='content'>5</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>3</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 11, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>3290 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>5731 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>−1, +1, +3, +4, +5</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.5</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 761 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>138 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>200 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>57.5 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−1.7·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Anders Gustaf Ekeberg in 1802</span>" },
Tungsten: {
name:
"<span class='title'>Element:</span> <span class='content'>Tungsten</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>W</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>74</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>183.84</span>",
group: "<span class='title'>Group:</span> <span class='content'>6</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>4</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 12, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>3695 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>6203 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>−2, -1, +1, +2, +3, +4, +5, +6</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.36</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 770 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>139 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>210 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>170 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−5.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Carl Wilhelm Scheele in 1781</span>" },
Rhenium: {
name:
"<span class='title'>Element:</span> <span class='content'>Rhenium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Re</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>75</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>186.21</span>",
group: "<span class='title'>Group:</span> <span class='content'>7</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>5</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 13, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>3459 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>5869 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>−3, -1, +1, +2, +3, +4, +5, +6, +7</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.9</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 760 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>137 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>47.9 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>antiferromagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−17.6·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Masataka Ogawa in 1908</span>" },
Osmium: {
name:
"<span class='title'>Element:</span> <span class='content'>Osmium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Os</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>76</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>190.23</span>",
group: "<span class='title'>Group:</span> <span class='content'>8</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>6</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 14, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>3306 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>5285 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>−2, -1, +1, +2, +3, +4, +5, +6, +7, +8</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.2</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 840 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>130 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>nan</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>87.6 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−14.6·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Smithson Tennant in 1803</span>" },
Iridium: {
name:
"<span class='title'>Element:</span> <span class='content'>Iridium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ir</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>77</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>192.22</span>",
group: "<span class='title'>Group:</span> <span class='content'>9</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>7</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 15, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2719 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>4701 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>−3, -1, +1, +2, +3, +4, +5, +6</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.20</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 880 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>135 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>202 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>147 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−5.7·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Smithson Tennant in 1803</span>" },
Platinum: {
name:
"<span class='title'>Element:</span> <span class='content'>Platinum</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Pt</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>78</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>195.08</span>",
group:
"<span class='title'>Group:</span> <span class='content'>10</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>9</sup> 6s<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 17, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2041 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>4098 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>2, 4, 5, 6</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.28</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 870 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>136 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>no data</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>71.6 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−3.1·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Used by pre-Columbian Americans</span>" },
Gold: {
name:
"<span class='title'>Element:</span> <span class='content'>Gold</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Au</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>79</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>196.97</span>",
group:
"<span class='title'>Group:</span> <span class='content'>11</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>10</sup> 6s<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 18, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1337.33 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3129 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>−1, +1, +2, +3, +5</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.54</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 890 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>144 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>166 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>315 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−5.86·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since prehistoric times</span>" },
Mercury: {
name:
"<span class='title'>Element:</span> <span class='content'>Mercury</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Hg</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>80</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>200.59</span>",
group:
"<span class='title'>Group:</span> <span class='content'>12</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>10</sup> 6s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 18, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Liquid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>234.32 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>629.88 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1, +2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.00</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1007.1 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>149 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>155 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>rhombohedral</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>8.30 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−2.8·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Thallium: {
name:
"<span class='title'>Element:</span> <span class='content'>Thallium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Tl</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>81</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>204.38</span>",
group:
"<span class='title'>Group:</span> <span class='content'>13</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Post-transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>10</sup> 6s<sup>2</sup> 6p<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 18, 3</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>577 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1746 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1, +3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.62</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 589.4 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>145 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>196 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>46.1 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−0.20·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by William Crookes in 1861</span>" },
Lead: {
name:
"<span class='title'>Element:</span> <span class='content'>Lead</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Pb</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>82</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>207.2</span>",
group:
"<span class='title'>Group:</span> <span class='content'>14</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Post-transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>10</sup> 6s<sup>2</sup> 6p<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 18, 4</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>600.61 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2022 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +4</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.87</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 715.6 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>146 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>202 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>35.9 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−19.0·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Bismuth: {
name:
"<span class='title'>Element:</span> <span class='content'>Bismuth</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Bi</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>83</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>208.98</span>",
group:
"<span class='title'>Group:</span> <span class='content'>15</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Post-transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>10</sup> 6s<sup>2</sup> 6p<sup>3</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 18, 5</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>544.55 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1837 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +5</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.02</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 703 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>148 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>207 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>rhombohedral</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>7.97 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−16.2·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Known since ancient times</span>" },
Polonium: {
name:
"<span class='title'>Element:</span> <span class='content'>Polonium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Po</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>84</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>209</span>",
group:
"<span class='title'>Group:</span> <span class='content'>16</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Metalloid</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>10</sup> 6s<sup>2</sup> 6p<sup>4</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 18, 6</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>527 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1235 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +4, +6</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.00</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 812.1 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>140 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>197 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>rhombohedral</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>20 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>diamagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>−34.8·10−6 cm<sup>3</sup>/mol</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Marie Curie and Pierre Curie in 1898</span>" },
Astatine: {
name:
"<span class='title'>Element:</span> <span class='content'>Astatine</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>At</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>85</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>210</span>",
group:
"<span class='title'>Group:</span> <span class='content'>17</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Halogen</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>10</sup> 6s<sup>2</sup> 6p<sup>5</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 18, 7</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>575 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>610 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>−1, +1, +3, +5</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.2</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 919 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>150 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>202 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Dale R. Corson, Kenneth Ross MacKenzie, and Emilio Segrè in 1940</span>" },
Radon: {
name:
"<span class='title'>Element:</span> <span class='content'>Radon</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Rn</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>86</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>222</span>",
group:
"<span class='title'>Group:</span> <span class='content'>18</span>",
period:
"<span class='title'>Period:</span> <span class='content'>6</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Noble gas</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Xe] 4f<sup>14</sup> 5d<sup>10</sup> 6s<sup>2</sup> 6p<sup>6</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 18, 8</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Gas</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>202 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>211.5 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>0</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>2.2</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1037 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>150 pm</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>220 pm</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>3.61 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Friedrich Ernst Dorn in 1900</span>" },
Francium: {
name:
"<span class='title'>Element:</span> <span class='content'>Francium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Fr</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>87</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>223</span>",
group: "<span class='title'>Group:</span> <span class='content'>1</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Alkali metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 7s<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 18, 8, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>300 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>950 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.7</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 380 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Marguerite Perey in 1939</span>" },
Radium: {
name:
"<span class='title'>Element:</span> <span class='content'>Radium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ra</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>88</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>226</span>",
group: "<span class='title'>Group:</span> <span class='content'>2</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Alkaline earth metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>s-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 18, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1413 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>1413 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.9</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 509.3 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>18.6 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>nonmagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Marie Curie and Pierre Curie in 1898</span>" },
Actinium: {
name:
"<span class='title'>Element:</span> <span class='content'>Actinium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ac</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>89</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>227</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 6d<sup>1</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 18, 9, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1500 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3503 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.1</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 499 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>12 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Friedrich Oskar Giesel in 1902</span>" },
Thorium: {
name:
"<span class='title'>Element:</span> <span class='content'>Thorium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Th</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>90</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>232.0377</span>",
group: "<span class='title'>Group:</span> <span class='content'>4</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 6d<sup>2</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 18, 10, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2115 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>5061 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +3, +4</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 587 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>face-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>54.0 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Jöns Jakob Berzelius in 1828</span>" },
Protactinium: {
name:
"<span class='title'>Element:</span> <span class='content'>Protactinium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Pa</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>91</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>231.03588</span>",
group:
"<span class='title'>Group:</span> <span class='content'>15</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>2</sup> 6d<sup>1</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 20, 9, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1841 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>4300 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+5</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.5</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 568 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>body-centered cubic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>47.9 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by William Crookes in 1913</span>" },
Uranium: {
name:
"<span class='title'>Element:</span> <span class='content'>Uranium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>U</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>92</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>238.02891</span>",
group:
"<span class='title'>Group:</span> <span class='content'>16</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>3</sup> 6d<sup>1</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 21, 9, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1405.3 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>4404 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +4, +5, +6</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.38</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 597.6 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>orthorhombic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>27.5 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Martin Heinrich Klaproth in 1789</span>" },
Neptunium: {
name:
"<span class='title'>Element:</span> <span class='content'>Neptunium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Np</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>93</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>237</span>",
group:
"<span class='title'>Group:</span> <span class='content'>15</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>4</sup> 6d<sup>1</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 22, 9, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>917 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>4273 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +4, +5, +6, +7</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.36</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 604.5 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>orthorhombic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>6.3 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Edwin McMillan and Philip H. Abelson in 1940</span>" },
Plutonium: {
name:
"<span class='title'>Element:</span> <span class='content'>Plutonium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Pu</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>94</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>244</span>",
group:
"<span class='title'>Group:</span> <span class='content'>16</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>6</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 24, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>912 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>3505 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +4, +5, +6, +7</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.28</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 584.7 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>monoclinic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>6.74 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Glenn T. Seaborg, Ralph A. James, Leon O. Morgan, and Albert Ghiorso in 1940</span>" },
Americium: {
name:
"<span class='title'>Element:</span> <span class='content'>Americium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Am</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>95</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>243</span>",
group:
"<span class='title'>Group:</span> <span class='content'>15</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>7</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 25, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1449 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>2880 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +3, +4, +5, +6</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 578 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>10 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Glenn T. Seaborg, Ralph A. James, Leon O. Morgan, and Albert Ghiorso in 1944</span>" },
Curium: {
name:
"<span class='title'>Element:</span> <span class='content'>Curium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Cm</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>96</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>247</span>",
group:
"<span class='title'>Group:</span> <span class='content'>16</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>7</sup> 6d<sup>1</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 25, 9, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1613 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +4</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 581 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>double hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>13.3 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944</span>" },
Berkelium: {
name:
"<span class='title'>Element:</span> <span class='content'>Berkelium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Bk</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>97</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>247</span>",
group:
"<span class='title'>Group:</span> <span class='content'>16</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>9</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 27, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1259 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3, +4</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 601 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>orthorhombic</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>10.5 W/(m·K)</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>paramagnetic</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1949</span>" },
Californium: {
name:
"<span class='title'>Element:</span> <span class='content'>Californium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Cf</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>98</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>251</span>",
group:
"<span class='title'>Group:</span> <span class='content'>18</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>10</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 28, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1173 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +3, +4</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 608 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Albert Ghiorso, Glenn T. Seaborg, Ralph A. James, and Torbjørn Sikkeland in 1950</span>" },
Einsteinium: {
name:
"<span class='title'>Element:</span> <span class='content'>Einsteinium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Es</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>99</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>252</span>",
group:
"<span class='title'>Group:</span> <span class='content'>18</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>11</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 29, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1133 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 619 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>hexagonal close-packed</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Albert Ghiorso, Glenn T. Seaborg, Ralph A. James, and Gregory R. Choppin in 1952</span>" },
Fermium: {
name:
"<span class='title'>Element:</span> <span class='content'>Fermium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Fm</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>100</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>257</span>",
group:
"<span class='title'>Group:</span> <span class='content'>18</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>12</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 30, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1800 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 627 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Albert Ghiorso, Glenn T. Seaborg, Ralph A. James, and Stanley G. Thompson in 1952</span>" },
Mendelevium: {
name:
"<span class='title'>Element:</span> <span class='content'>Mendelevium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Md</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>101</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>258</span>",
group:
"<span class='title'>Group:</span> <span class='content'>18</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>13</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 31, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1100 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 635 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Albert Ghiorso, Glenn T. Seaborg, Ralph A. James, and M. Albert in 1955</span>" },
Nobelium: {
name:
"<span class='title'>Element:</span> <span class='content'>Nobelium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>No</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>102</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>259</span>",
group:
"<span class='title'>Group:</span> <span class='content'>18</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Actinide</span>",
block:
"<span class='title'>Block:</span> <span class='content'>f-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 8, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1100 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 642 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Albert Ghiorso, Glenn T. Seaborg, Ralph A. James, and Leon O. Morgan in 1957</span>" },
Lawrencium: {
name:
"<span class='title'>Element:</span> <span class='content'>Lawrencium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Lr</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>103</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>262</span>",
group: "<span class='title'>Group:</span> <span class='content'>3</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>1</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 8, 3</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>1900 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 470 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Discovered by Albert Ghiorso, Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1961</span>" },
Rutherfordium: {
name:
"<span class='title'>Element:</span> <span class='content'>Rutherfordium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Rf</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>104</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>267</span>",
group: "<span class='title'>Group:</span> <span class='content'>4</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>2</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 10, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>2400 K</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>5800 K</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+4</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 580 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>Synthesized by bombarding plutonium-242 with carbon ions in 1969</span>" },
Dubnium: {
name:
"<span class='title'>Element:</span> <span class='content'>Dubnium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Db</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>105</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>268</span>",
group: "<span class='title'>Group:</span> <span class='content'>5</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>3</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 11, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+5</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 663 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the Joint Institute for Nuclear Research (JINR) in 1968</span>" },
Seaborgium: {
name:
"<span class='title'>Element:</span> <span class='content'>Seaborgium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Sg</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>106</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>271</span>",
group: "<span class='title'>Group:</span> <span class='content'>6</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>4</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 12, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+6</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 761 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the Joint Institute for Nuclear Research (JINR) in 1974</span>" },
Bohrium: {
name:
"<span class='title'>Element:</span> <span class='content'>Bohrium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Bh</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>107</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>270</span>",
group: "<span class='title'>Group:</span> <span class='content'>7</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>5</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 13, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+7</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 740 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the Joint Institute for Nuclear Research (JINR) in 1976</span>" },
Hassium: {
name:
"<span class='title'>Element:</span> <span class='content'>Hassium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Hs</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>108</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>277</span>",
group: "<span class='title'>Group:</span> <span class='content'>8</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>6</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 14, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+8, +6, +4, +2, 0</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 870 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the German Institute for Heavy Ion Research (Gesellschaft für Schwerionenforschung, GSI) in 1984</span>" },
Meitnerium: {
name:
"<span class='title'>Element:</span> <span class='content'>Meitnerium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Mt</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>109</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>276</span>",
group: "<span class='title'>Group:</span> <span class='content'>9</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>7</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 15, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+9, +8, +6, +4, +3, +1, -1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 800 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the Gesellschaft für Schwerionenforschung (GSI) in 1982</span>" },
Darmstadtium: {
name:
"<span class='title'>Element:</span> <span class='content'>Darmstadtium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ds</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>110</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>281</span>",
group:
"<span class='title'>Group:</span> <span class='content'>10</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>8</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 16, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+8, +6, +4, +2, 0</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.1</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1100 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the Gesellschaft für Schwerionenforschung (GSI) in 1994</span>" },
Roentgenium: {
name:
"<span class='title'>Element:</span> <span class='content'>Roentgenium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Rg</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>111</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>280</span>",
group:
"<span class='title'>Group:</span> <span class='content'>11</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>9</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 17, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+5, +3, 0</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.9</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1100 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the Gesellschaft für Schwerionenforschung (GSI) in 1994</span>" },
Copernicium: {
name:
"<span class='title'>Element:</span> <span class='content'>Copernicium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Cn</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>112</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>285</span>",
group:
"<span class='title'>Group:</span> <span class='content'>12</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>d-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>10</sup> 7s<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 18, 2</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +4</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.9</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 1150 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the Gesellschaft für Schwerionenforschung (GSI) in 1996</span>" },
Nihonium: {
name:
"<span class='title'>Element:</span> <span class='content'>Nihonium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Nh</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>113</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>284</span>",
group:
"<span class='title'>Group:</span> <span class='content'>13</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Post-transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>10</sup> 7s<sup>2</sup> 7p<sup>1</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 18, 1</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.9</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 700 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the RIKEN collaboration in 2003</span>" },
Flerovium: {
name:
"<span class='title'>Element:</span> <span class='content'>Flerovium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Fl</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>114</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>289</span>",
group:
"<span class='title'>Group:</span> <span class='content'>14</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Post-transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>10</sup> 7s<sup>2</sup> 7p<sup>2</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 18, 4</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+4, +2, 0</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>1.3</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 760 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the Joint Institute for Nuclear Research (JINR) and Lawrence Livermore National Laboratory (LLNL) in 1998</span>" },
Moscovium: {
name:
"<span class='title'>Element:</span> <span class='content'>Moscovium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Mc</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>115</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>290</span>",
group:
"<span class='title'>Group:</span> <span class='content'>15</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Post-transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>10</sup> 7s<sup>2</sup> 7p<sup>3</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 18, 5</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1, +3</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.9</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 800 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the Joint Institute for Nuclear Research (JINR) and Lawrence Livermore National Laboratory (LLNL) in 2003</span>" },
Livermorium: {
name:
"<span class='title'>Element:</span> <span class='content'>Livermorium</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Lv</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>116</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>293</span>",
group:
"<span class='title'>Group:</span> <span class='content'>16</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Post-transition metal</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>10</sup> 7s<sup>2</sup> 7p<sup>4</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 18, 6</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+2, +4, +6</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.9</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 870 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the Joint Institute for Nuclear Research (JINR) and Lawrence Livermore National Laboratory (LLNL) in 2000</span>" },
Tennessine: {
name:
"<span class='title'>Element:</span> <span class='content'>Tennessine</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Ts</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>117</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>294</span>",
group:
"<span class='title'>Group:</span> <span class='content'>17</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Halogen</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>10</sup> 7s<sup>2</sup> 7p<sup>5</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 18, 7</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1, +3, +5</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.9</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 870 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the Joint Institute for Nuclear Research (JINR) and Oak Ridge National Laboratory (ORNL) in 2010</span>" },
Oganesson: {
name:
"<span class='title'>Element:</span> <span class='content'>Oganesson</span>",
symbol:
"<span class='title'>Symbol:</span> <span class='content'>Og</span>",
"atomic-number":
"<span class='title'>Atomic number:</span> <span class='content'>118</span>",
"atomic-weight":
"<span class='title'>Atomic weight:</span> <span class='content'>294</span>",
group:
"<span class='title'>Group:</span> <span class='content'>18</span>",
period:
"<span class='title'>Period:</span> <span class='content'>7</span>",
category:
"<span class='title'>Category:</span> <span class='content'>Noble gas</span>",
block:
"<span class='title'>Block:</span> <span class='content'>p-block</span>",
"electron-configuration":
"<span class='title'>Electron configuration:</span> <span class='content'>[Rn] 5f<sup>14</sup> 6d<sup>10</sup> 7s<sup>2</sup> 7p<sup>6</sup></span>",
"electron-per-shell":
"<span class='title'>Electrons per shell:</span> <span class='content'>2, 8, 18, 32, 32, 18, 8</span>",
"phase-at-STP":
"<span class='title'>Phase at STP:</span> <span class='content'>Solid</span>",
"melting-point":
"<span class='title'>Melting point:</span> <span class='content'>unknown</span>",
"boiling-point":
"<span class='title'>Boiling point:</span> <span class='content'>unknown</span>",
"oxidation-states":
"<span class='title'>Oxidation states:</span> <span class='content'>+1, +3, +5</span>",
electronegativity:
"<span class='title'>Pauling scale:</span> <span class='content'>0.9</span>",
"ionization-energy":
"<span class='title'>Ionization energy:</span> <span class='content'>1st: 830 kJ/mol</span>",
"covalent-radius":
"<span class='title'>Covalent radius:</span> <span class='content'>unknown</span>",
"van-der-waals-radius":
"<span class='title'>Van der Waals radius:</span> <span class='content'>unknown</span>",
"crystal-structure":
"<span class='title'>Crystal structure:</span> <span class='content'>unknown</span>",
"thermal-conductivity":
"<span class='title'>Thermal conductivity:</span> <span class='content'>unknown</span>",
"magnetic-order":
"<span class='title'>Magnetic order:</span> <span class='content'>unknown</span>",
"magnetic-susceptibility":
"<span class='title'>Magnetic susceptibility:</span> <span class='content'>unknown</span>",
miscellaneous:
"<span class='title'>Miscellaneous:</span> <span class='content'>First synthesized by the Joint Institute for Nuclear Research (JINR) and Lawrence Livermore National Laboratory (LLNL) in 2002</span>" } };
let periodList = document.querySelectorAll(".period__item"),
groupList = document.querySelectorAll(".group__item"),
elementList = document.querySelectorAll(".element"),
legendList = document.querySelectorAll(".legend-box"),
temperatureSlider = document.querySelector(".temperature__inputs__slider"),
resetTemperatureButton = document.querySelector(".reset__temperature"),
lanthanoidBox = document.querySelector(`[data-element-name='Lanthanoids']`),
actinoidBox = document.querySelector(`[data-element-name='Actinoids']`),
modalBox = document.querySelector(".modal"),
modalContent = document.querySelector(".modal__content"),
modalProperties = document.querySelector(".modal__content__properties"),
modalClose = document.querySelector(".modal__close"),
isOpen = false,
elementClicked;
window.addEventListener("scroll", () => {
const scrollY = window.scrollY > 0 ? true : false,
scrollX = window.scrollX > 0 ? true : false,
period = document.querySelector(".period__list"),
group = document.querySelector(".group__list");
if (scrollX) {
addClass("--is-fixed", period, 0);
period.style.left = `${window.scrollX}px`;
} else {
removeClass("--is-fixed", period, 0);
period.style.left = 0;
}
if (scrollY) {
addClass("--is-fixed", group, 0);
} else {
removeClass("--is-fixed", group, 0);
}
});
modalClose.addEventListener("click", closeModal);
modalBox.addEventListener("click", closeModal);
document.addEventListener("keydown", event => {
if (event.keyCode === 27 && isOpen) {
closeModal(event);
}
});
actinoidBox.addEventListener("mouseenter", self => {
const dataActinoids = self.target.getAttribute("data-element-type"),
dataToSearch = "type";
highlightElement(dataActinoids, dataToSearch);
});
actinoidBox.addEventListener("mouseleave", () => {
equalizeElement();
});
lanthanoidBox.addEventListener("mouseenter", self => {
const dataLantanoids = self.target.getAttribute("data-element-type"),
dataToSearch = "type";
highlightElement(dataLantanoids, dataToSearch);
});
lanthanoidBox.addEventListener("mouseleave", () => {
equalizeElement();
});
resetTemperatureButton.addEventListener("click", () => {
const dataToSearch = ["melting-point", "boiling-point"];
temperatureSlider.value = STP;
setNewTemperature(STP);
highlightElement(STP, dataToSearch);
removeClass("--is-visible", resetTemperatureButton, 0);
});
temperatureSlider.addEventListener("input", self => {
const currentValue = Number(self.target.value),
dataToSearch = ["melting-point", "boiling-point"];
if (
currentValue !== STP &&
!resetTemperatureButton.classList.contains("--is-visible"))
{
addClass("--is-visible", resetTemperatureButton, 0);
} else if (currentValue === STP) {
removeClass("--is-visible", resetTemperatureButton, 0);
}
setNewTemperature(currentValue);
highlightElement(currentValue, dataToSearch);
});
Array.from(elementList).forEach(elementItem => {
elementItem.addEventListener("click", () => {
if (isLanthanoidOrActinoid(elementItem)) {
elementClicked = elementItem;
elementName = elementClicked.getAttribute("data-element-name");
modalAnimation(elementItem);
createModalContent(elementName);
}
});
});
Array.from(legendList).forEach(legendItem => {
legendItem.addEventListener("mouseenter", self => {
let legendData, dataToSearch;
if (legendItem.getAttribute("data-element-type")) {
legendData = self.target.getAttribute("data-element-type");
dataToSearch = "type";
} else {
legendData = self.target.getAttribute("data-element-state");
dataToSearch = "state";
}
highlightElement(legendData, dataToSearch);
});
legendItem.addEventListener("mouseleave", () => {
equalizeElement();
});
});
Array.from(periodList).forEach(periodItem => {
periodItem.addEventListener("mouseenter", self => {
const periodNumber = Number(self.target.firstChild.innerHTML),
dataToSearch = "period";
highlightElement(periodNumber, dataToSearch);
});
periodItem.addEventListener("mouseleave", () => {
equalizeElement();
});
});
Array.from(groupList).forEach(groupItem => {
groupItem.addEventListener("mouseenter", self => {
const groupNumber = Number(self.target.firstChild.innerHTML),
dataToSearch = "group";
highlightElement(groupNumber, dataToSearch);
});
groupItem.addEventListener("mouseleave", () => {
equalizeElement();
});
});
function equalizeElement() {
let index = 0;
Array.from(elementList).forEach(elementItem => {
removeClass("--is-active", elementItem, index);
index++;
});
}
function highlightElement(dataElement, dataToSearch) {
let index = 0;
Array.from(elementList).forEach(elementItem => {
let dataFromElement = elementItem.getAttribute(
`data-element-${dataToSearch}`);
if (dataToSearch === "group" || dataToSearch === "period") {
dataFromElement = Number(dataFromElement);
}
if (dataToSearch.length === 2) {
let meltingPoint = Number(
elementItem.getAttribute(`data-element-${dataToSearch[0]}`)),
boilingPoint = Number(
elementItem.getAttribute(`data-element-${dataToSearch[1]}`));
if (!isNaN(meltingPoint) && !isNaN(boilingPoint)) {
if (dataElement < meltingPoint) {
elementItem.setAttribute("data-element-state", "solid");
} else if (dataElement < boilingPoint) {
if (isLanthanoidOrActinoid(elementItem)) {
elementItem.setAttribute("data-element-state", "liquid");
}
} else {
elementItem.setAttribute("data-element-state", "gas");
}
} else if (isNaN(boilingPoint)) {
let elementState = "unknown";
if (dataElement < meltingPoint) {
elementState = "solid";
}
elementItem.setAttribute("data-element-state", elementState);
}
}
if (dataElement === dataFromElement) {
addClass("--is-active", elementItem, index);
}
index++;
});
}
function createModalContent(elementName) {
Object.keys(ELEMENT_MODAL_DATA[elementName]).forEach(
(key) =>
modalProperties.innerHTML += `<p class='element-property element-${key} flex-column-wrap'>${ELEMENT_MODAL_DATA[elementName][key]}</p>`);
}
function removeModalContent() {
modalProperties.innerHTML = "";
}
function modalAnimation(self) {
let selfProperties = self.getBoundingClientRect(),
modalProperties = modalContent.getBoundingClientRect(),
tooltip = self.querySelector(".tooltip"),
translateX,
translateY,
scale,
positionX = window.innerWidth / 2,
positionY = window.innerHeight / 2;
addClass("--is-hidden", tooltip, 0);
addClass("--is-triggered", self, 0);
addClass("modal__background", modalBox, 0);
scale = modalProperties.width / 100;
translateX = Math.round(
positionX - selfProperties.left - selfProperties.width / 2);
translateY = Math.round(
positionY - selfProperties.top - selfProperties.height / 2);
self.style.transform = `translate(${translateX}px, ${translateY}px) scale(${scale})`;
window.requestAnimationFrame(() => {
openModal();
});
}
function openModal() {
if (!isOpen) {
const content = modalBox.querySelector(".modal__content");
addClass("--is-visible", modalBox, 0);
addClass("--is-visible", content, 75);
content.addEventListener("transitionend", hideContent(content), false);
isOpen = true;
}
}
function hideContent(content) {
content.removeEventListener("transitionend", hideContent, false);
}
function closeModal(event) {
event.preventDefault();
event.stopImmediatePropagation();
const target = event.target,
tooltip = elementClicked.querySelector(".tooltip");
if (
isOpen && target.classList.contains("modal__background") ||
target.classList.contains("modal__close") ||
event.keyCode === 27)
{
removeClass("--is-visible", modalBox, 0);
removeClass("--is-visible", modalContent, 0);
elementClicked.removeAttribute("style");
removeClass("--is-triggered", elementClicked, 0);
removeClass("modal__background", modalBox, 0);
removeClass("--is-hidden", tooltip, 0);
removeModalContent();
elementClicked = "";
isOpen = false;
}
}
function setNewTemperature(currentValue) {
const kelvinOutput = document.querySelector(".temperature__inputs__result"),
celsiusOutput = document.querySelector(".celsius"),
farenheitOutput = document.querySelector(".farenheit");
kelvinOutput.innerHTML = `${currentValue} K`;
celsiusOutput.innerHTML = `${currentValue - 273}ºC`;
farenheitOutput.innerHTML = `${
Math.round((currentValue * 9 / 5 - 460) * 100) / 100
}ºF`;
}
function addClass(className, element, index) {
setTimeout(() => {
element.classList.add(className);
}, index * DELAY);
}
function removeClass(className, element, index) {
setTimeout(() => {
element.classList.remove(className);
}, index * DELAY);
}
function isLanthanoidOrActinoid(elementItem) {
return (
elementItem.getAttribute("data-element-name") !== "Lanthanoids" &&
elementItem.getAttribute("data-element-name") !== "Actinoids");
}
That’s all! Hopefully, you have successfully created your Periodic Table of Elements HTML Code.